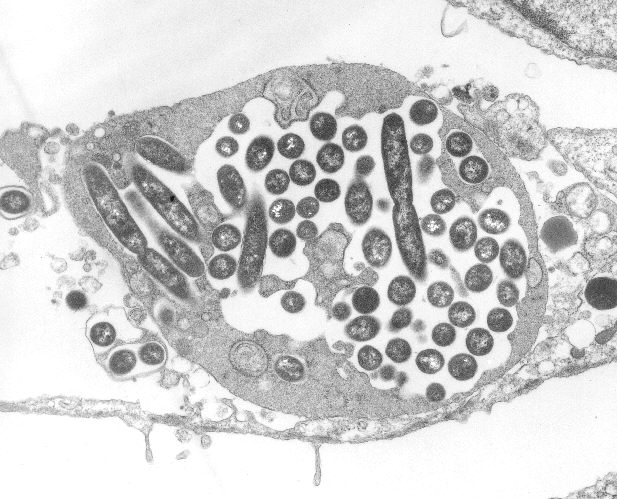
Commentary by Elizabeth Hackett MD, PGY-3
On July 25th, 2007, the NYC Department of Health released an advisory requesting that all New York City physicians maintain a high index of suspicion for Legionnaires’ disease in patients presenting …


Commentary by Elizabeth Hackett MD, PGY-3
On July 25th, 2007, the NYC Department of Health released an advisory requesting that all New York City physicians maintain a high index of suspicion for Legionnaires’ disease in patients presenting …
Back in December we reported on the FDA cautioning practioners about the use of gadolinium (an mri contrast agent) in patients with chronic kidney disease. The FDA is now requesting a black box warning stating “that patients with severe kidney insufficiency who receive gadolinium-based agents are at risk for developing a debilitating, …
 Commentary By: Marshall Fordyce, PGY-3
Commentary By: Marshall Fordyce, PGY-3
Now that the dust has settled in Texas and Virginia, let’s clarify the role of the human papilloma virus (HPV) vaccine in our clinics. An excellent article in last week’s JAMA by its Editor-In-Chief, Dr. Catherine DeAngelis, and Lawrence Gostin, …
 This week, olives from several different companies were found to contain Clostridium Botulinum. No cases of botulism have been reported to date, but this is an opportunity to review the pertinent clinical findings.
This week, olives from several different companies were found to contain Clostridium Botulinum. No cases of botulism have been reported to date, but this is an opportunity to review the pertinent clinical findings.
Botulism is caused by exposure to the botulinum neurotoxin in …
Commentary By: Steven Sedlis, MD Associate Professor of Medicine, Chief, Division of Cardiology Manhattan Veterans Administration Medical Center
The 56th annual scientific session of the American College of Cardiology was held in New Orleans on March 24-27. The site of the meeting had …
 Commentary By: Josh Olstein, PGY-3
Commentary By: Josh Olstein, PGY-3
Earlier this month the FDA approved Tekturna (aliskiren) the first drug in a novel class of antihypertensives that work by directly inhibiting renin. While Novartis has yet to release pricing information, don’t expect to see this new …
Commentary by Cara Litvin, PGY-3
The results of one of the more remarkable studies from the meeting of the American College of Cardiology were presented on Monday, along with the simultaneous early publishing of the study online in the New England Journal of Medicine. …
 The debate about the ethically questionable relationship between physicians and the pharmaceutical industry opened up again this morning on the front page of the New York Times. Although the article is heavy on interview and anecdote and a little short on evidence, …
The debate about the ethically questionable relationship between physicians and the pharmaceutical industry opened up again this morning on the front page of the New York Times. Although the article is heavy on interview and anecdote and a little short on evidence, …