 By Michael Lee, MD
By Michael Lee, MD
Peer Reviewed
The human taste bud has become increasingly accustomed to the Japanese invention of the early 20th century: monosodium glutamate, better known as MSG. Its basic component, glutamate, is a …

 By Michael Lee, MD
By Michael Lee, MD
Peer Reviewed
The human taste bud has become increasingly accustomed to the Japanese invention of the early 20th century: monosodium glutamate, better known as MSG. Its basic component, glutamate, is a …
 By Jennifer Zhu
By Jennifer Zhu
Peer Reviewed
After the elementary school shooting in Newtown, CT in December 2012 that left 20 children and 6 adults dead, the country reacted as it had following the July …
 By Aaron Smith, MD
By Aaron Smith, MD
Peer Reviewed
First introduced in the late 1980s, proton pump inhibitors (PPIs) have revolutionized the treatment of gastric acid-related disorders and have been described as a miracle drug by patients and …
 Please enjoy this post from the archives dated September 21, 2011
Please enjoy this post from the archives dated September 21, 2011
By Alon Mass
Faculty Peer Reviewed
The overlap between religion and medicine is ancient. On a recent medical volunteer trip to India I met a medical student who proudly wore a school …
 By Theresa Sumberac, MD
By Theresa Sumberac, MD
Peer Reviewed
The 2008 US Census Bureau reported that 14 to 16 percent of the adult population enjoyed crossword puzzles and that half of them played crossword puzzles …
 Please enjoy this post from the archives dated September 8, 2011
Please enjoy this post from the archives dated September 8, 2011
By David Altszuler, Class of 2012
Faculty Peer Reviewed
An empiric association between occult malignancy and thrombophlebitis has been recognized since Trousseau first reported the syndrome in 1865.[1] The mechanism by …
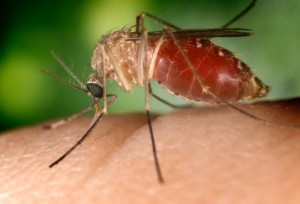 By Julian Horwitz
By Julian Horwitz
Peer Reviewed
As of mid-August 2012, the CDC had reported 1118 cases of West Nile virus (WNV) infections and 41 related deaths, which, pro rata, made 2012 the most prolific year for …
 By Nicole A. Lamparello, MD and Molly Somberg, MD, MPA
By Nicole A. Lamparello, MD and Molly Somberg, MD, MPA
Peer Reviewed
You hear it wherever you eat, whether at the deli ordering a breakfast sandwich or at the diner for Sunday brunch, “Egg whites …