 By Ian Henderson, MD
By Ian Henderson, MD
Peer Reviewed
An apple a day keeps the doctor away, right? Viewed by the public to be so healthy as to prevent doctor’s visits, the apple may be losing its health …

 By Ian Henderson, MD
By Ian Henderson, MD
Peer Reviewed
An apple a day keeps the doctor away, right? Viewed by the public to be so healthy as to prevent doctor’s visits, the apple may be losing its health …
 By Shyam Amin, MD
By Shyam Amin, MD
Peer reviewed
In the news…
Congratulations to the New England Patriots and their fans for a thrilling Super Bowl victory this past week. A full ten years since their last Super Bowl …
 By Kelley Coffman, M.D.
By Kelley Coffman, M.D.
Peer Reviewed
The anti-vaccine movement appears to be gathering steam on social media and major media networks despite a growing outbreak of measles spreading to 8 states, according to California …
 By Meng Chen, MD
By Meng Chen, MD
Peer Reviewed
Last Friday, in an announcement of hope and optimism, Reuters quoted a senior health official from Liberia as reporting only five remaining confirmed cases of Ebola in Liberia, which …
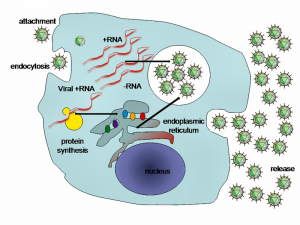 By Jovan Begovic, MD
By Jovan Begovic, MD
Peer Reviewed
Last Friday, Aetna and Gilead Sciences, the well-known maker of Sovaldi, the “$1000-a-pill” treatment for Hepatitis C, reached a discount agreement that will allow Sovaldi to populate Aetna’s …
 By Jessica Yee, MD
By Jessica Yee, MD
Peer Reviewed
Welcome to the first PrimeCuts of 2015!
Welcome to the first PrimeCuts of 2015! The year started with a shout-out from 200 years ago in …
 Sick and tired of all those top 10 lists? We beg your indulgence and hope you will tolerate just one more, the Clinical Correlations Top 10 …
Sick and tired of all those top 10 lists? We beg your indulgence and hope you will tolerate just one more, the Clinical Correlations Top 10 …
 By: Kristina Cieslak
By: Kristina Cieslak
This Wednesday, President Obama announced plans to “end an outdated approach, that for decades, has failed to advance our interests,” ordering restoration of full diplomatic relations …