 By Antony Q. Pham, Pharm.D. and Reena M. Tejura, Pharm.D.
By Antony Q. Pham, Pharm.D. and Reena M. Tejura, Pharm.D.
Faculty Peer Reviewed
Recent publications have described a potential drug interaction between clopidogrel (Plavix®) and proton pump inhibitors (PPIs). Several retrospective …

 By Antony Q. Pham, Pharm.D. and Reena M. Tejura, Pharm.D.
By Antony Q. Pham, Pharm.D. and Reena M. Tejura, Pharm.D.
Faculty Peer Reviewed
Recent publications have described a potential drug interaction between clopidogrel (Plavix®) and proton pump inhibitors (PPIs). Several retrospective …
 Marisa Mizus
Marisa Mizus
Faculty peer reviewed
Clopidogrel (Plavix) has been the standard of care for patients with coronary artery disease following percutaneous coronary intervention (PCI) for the past decade. Although it is …

John Cruz
Faculty peer reviewed
A number of studies have shown that zolpidem (Ambien), the most commonly prescribed sleep-inducing medication on the market, can produce uncontrollable nocturnal eating behavior …
 Kanika Ballani, Pharm.D.
Kanika Ballani, Pharm.D.
Diana Hubulasvili, Pharm.D.
Developed by Amylin Pharmaceuticals, Exenatide (Byetta®) is an incretin mimetic that is used as an adjunctive therapy with metformin, a sulfonylurea or a thiazolidinedione to improve glycemic control …
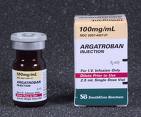
Frederick Gandolfo, MD
Case: An 85 year-old woman admitted to the hospital with pneumonia and after a prolonged hospital course developed heparin-induced thrombocytopenia (HIT). She is currently …

Commentary by Adi Diab MD, PGY-3
This past week on January 21th, Medical Grand Rounds was given by guest speaker Dr. John Griffin who shared his knowledge of the role of Activate Protein C …
 Commentary by Rachana Jani MD, PGY-2
Commentary by Rachana Jani MD, PGY-2
As recently reported in ShortCuts, Byetta recently made headlines after the suggestion of a mortality benefit for patients taking the drug in a small subset of the Accord study. So is this the new golden drug for …