 By Allison Harrington, MD
By Allison Harrington, MD
Peer Reviewed
Learning Objectives:
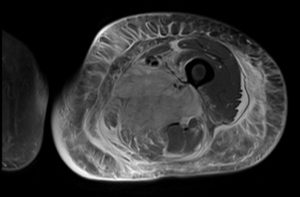
1) When should diabetic myonecrosis be suspected?
2) What are the diagnostic criteria for diabetic myonecrosis? What is the pathophysiology?
3) What is the management of diabetic myonecrosis? How can it be …

 By Eric Jeffrey Nisenbaum, MD
By Eric Jeffrey Nisenbaum, MD
 By Robert Mocharla, MD
By Robert Mocharla, MD By Karin Katz, MD
By Karin Katz, MD By Austin Peters, MD
By Austin Peters, MD By Tracey Liebman
By Tracey Liebman Class act is a feature of Clinical Correlations written by NYU 3rd and 4th year medical students. Prior to publication, each commentary is thoroughly reviewed for content by a faculty member.
Class act is a feature of Clinical Correlations written by NYU 3rd and 4th year medical students. Prior to publication, each commentary is thoroughly reviewed for content by a faculty member.