 By Olivia Descorbeth
By Olivia Descorbeth
Peer Reviewed
As individuals advance in age, they tend to accumulate medical conditions that require a bevy of pharmaceutical treatments to manage. As a result, polypharmacy, generally defined as the use of five …

 By Olivia Descorbeth
By Olivia Descorbeth
Peer Reviewed
As individuals advance in age, they tend to accumulate medical conditions that require a bevy of pharmaceutical treatments to manage. As a result, polypharmacy, generally defined as the use of five …
 By Jessica K Qiu
By Jessica K Qiu
Peer Reviewed
In 1998, there were 34 million adults aged 65 years or older in the US.1 By 2030, that number is expected to double.1 This dramatic increase in the …
 By Michael Nguyen
By Michael Nguyen
Peer Reviewed
Polypharmacy has been defined as the use of multiple unnecessary medications, the use of more medications than is clinically warranted or indicated, or the use of unnecessary, …
 By Carl Preiksaitis
By Carl Preiksaitis
Peer Reviewed
The term “sarcopenia” was introduced in 1989 to characterize the loss of muscle mass that occurs as a consequence of advancing age.1 Use of the term has …
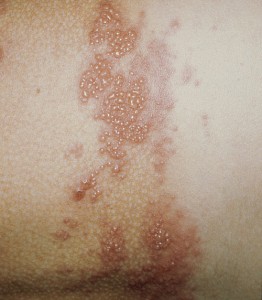 By Zachary Elkin
By Zachary Elkin
Faculty Peer Reviewed
There are more than a million cases of herpes zoster (HZ) in the US annually [1-3]. The incidence of HZ, or shingles, has been rising in the US since …
 Please enjoy this post from the archives dated January 30, 2010.
Please enjoy this post from the archives dated January 30, 2010.
Mark D. Schwartz and Julia Hyland Bruno
Jeanne Calment rode her bicycle until age 100, quit smoking at 117, and died in 1977 at 122 years of age in Arles, France. This …
 By Courtney Cunningham, MD
By Courtney Cunningham, MD
Faculty Peer Reviewed
As the world population ages, enormous resources will be required to adequately care for persons suffering from Alzheimer’s disease. The disease is the fifth …
 Gilda Boroumand, MS4
Gilda Boroumand, MS4
Faculty Peer Reviewed
Chronic insomnia, defined as difficulty with the initiation, maintenance, duration, and quality of sleep for at least one month, is a common complaint with significant …