 By Nicole A. Lamparello, MD and Molly Somberg, MD, MPA
By Nicole A. Lamparello, MD and Molly Somberg, MD, MPA
Peer Reviewed
Please enjoy this post from the archives dated February 27, 2014
You hear it wherever you eat, whether at the deli ordering a …

 By Nicole A. Lamparello, MD and Molly Somberg, MD, MPA
By Nicole A. Lamparello, MD and Molly Somberg, MD, MPA
Peer Reviewed
Please enjoy this post from the archives dated February 27, 2014
You hear it wherever you eat, whether at the deli ordering a …
 Please enjoy this post from the archives dated February 5, 2014
Please enjoy this post from the archives dated February 5, 2014
By Matthew A. Haber
Peer Reviewed
The following is a hypothetical example of a classic exam question that one might come across as a medical student:
A 50-year-old male presents to the emergency department with …
Please enjoy this post from the archives, dated January 29, 2014
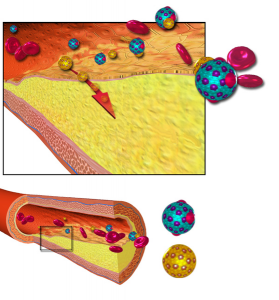 By Gregory Katz, MD
By Gregory Katz, MD
Peer Reviewed
As levels of HDL cholesterol increase, rates of heart disease go down. It’s this fact that has given HDL …

Please enjoy this post from the archives, date January 15, 2014
By Robert Mocharla, MD
Peer Reviewed
No. Sorry. Despite such reasonable excuses as – “I forgot my iPod”, “It’s pouring rain”, or “Game of Thrones …
Please enjoy this post from the archives dated January 10, 2014
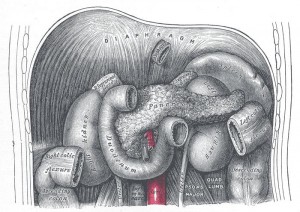 By Shivani K Patel, MD
By Shivani K Patel, MD
Peer reviewed
A 61-year old male with chronic epigastric discomfort presented to the emergency room with severe abdominal pain radiating …
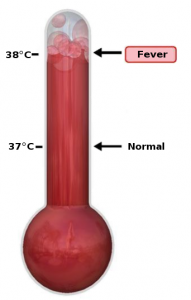
Please enjoy this post from the archives, dated November 20, 2013
By Fernando Franco Cuadrado, MD, Julia Hyland Bruno, MD and Mark D. Schwartz, MD
Faculty Peer Reviewed
When flu season returns, we will all see …

Please enjoy this post from the archives dated November 8, 2013
By Gregory Katz, MD
Faculty Peer Reviewed
Everyday in clinic, we tell our patients to choose foods low in saturated fat. Because these foods raise …
Please enjoy this post from the archives dated October 30, 2013
By Robert Joseph Fakheri, MD
Faculty Peer Reviewed
A 55 year-old male is recently diagnosed with systemic sarcoidosis. The patient is started on prednisone 40mg …