 By Ofole Mgbako, MD
By Ofole Mgbako, MD
Peer Reviewed
In July 2010, the much-anticipated “National HIV/AIDS Strategy for the United States” was released to the public. In its introduction, the president declared, “Our Nation is at a crossroads…unless we …

 By Ofole Mgbako, MD
By Ofole Mgbako, MD
Peer Reviewed
In July 2010, the much-anticipated “National HIV/AIDS Strategy for the United States” was released to the public. In its introduction, the president declared, “Our Nation is at a crossroads…unless we …
 By: Miguel A. Saldivar, MD
By: Miguel A. Saldivar, MD
Peer Reviewed
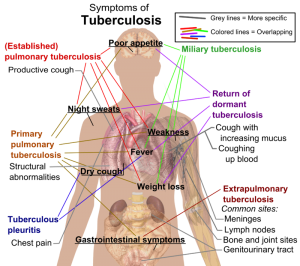
“Indeterminate.” Many clinicians have expressed frustration when reading this word on a Quantiferon-TB Gold test result. The obligate follow-up question is: what is the next best step? Repeat the …
 By M tanner
By M tanner
Many bacteria live in and on me—I’ve always known that. But when I learned that bacteria make up 90% of the cells in my body, it made me feel so sucio, so unclean.
I went through my day, realizing …
 By Rachel Kaplan Hoffmann, M.D., M.S.Ed., and Keith Hoffmann, J.D.
By Rachel Kaplan Hoffmann, M.D., M.S.Ed., and Keith Hoffmann, J.D.
Peer Reviewed
On December 6, 2013, a two-year-old boy living in southeastern Guinea became the first victim of the latest epidemic …
 By Nathan King
By Nathan King
Faculty Reviewed
Doctors are known to be some of the worst patients, and from personal experience I predict that medical students are not too far behind. That’s why when I finally found the time to take a proactive step in …
 By Luke O’Donnell, MD
By Luke O’Donnell, MD
Peer reviewed
Once formidable diseases, pneumonia, bacteremia, and meningitis are all now considered “bread-and-butter” internal medicine. Streptococcus pneumoniae is one of the major pathogens …
 By Pritha Subramanyam
By Pritha Subramanyam
Peer Reviewed
Mrs. CS is a 66-year-old Indian female who presents for a cardiology follow-up. The patient has a history of mitral regurgitation secondary to rheumatic fever she …
 By Theresa Sumberac, MD
By Theresa Sumberac, MD
Peer Reviewed
Antibiotic associated diarrhea is a common complication of antibiotic therapy, occurring in 5% to 39% of all patients receiving treatment. Nearly one third of these cases are attributed to …