Commentary by Muhammad Ghumman MD, PGY-3
Faculty Peer Reviewed
Clinical Case:
A 65 year old male with hypertension, iron deficiency anemia, and atrial fibrillation (not anticoagulated due to prior gastrointestinal bleed,) presents with new onset lower extremity edema, dyspnea on exertion, orthopnea, and profound fatigue. Physical exam is significant for jugular venous distention to 17 cm, bilateral basilar crackles on lung exam, 3+ pitting edema in the lower extremities to mid thighs, and guaiac positive brown stool. Labs are significant for hemoglobin of 5.4, normal kidney function, and mildly elevated troponin. Patient denies chest pain and is hemodynamically stable with normal vitals. Electrocardiogram shows new ST depressions in lateral leads. Transthoracic echocardiogram shows new segmental wall motion abnormalities along with a newly reduced ejection fraction of 30%. Capsule endoscopy shows non-bleeding angioectasias in the jejunum. The patient is started on medical therapy for congestive heart failure and presumed coronary artery disease (ACE-I, Beta blocker, diuretic) and transfused to hemoglobin goal of 10 with improvement in his symptoms of dyspnea and fatigue. Echocardiogram strongly suggests ischemic cardiomyopathy due to the presence of regional wall motion abnormalities and an assessment of the patient’s coronary anatomy is warranted. However, the medical team and cardiology consult are reluctant to perform cardiac catheterization due to the risk of bleeding in a severely anemic patient who may require coronary artery stenting and subsequent long term anticoagulation. What other modalities are available for assessing this patient’s new cardiomyopathy and coronary anatomy?
Introduction:
Cardiac magnetic resonance imaging (CMR) is a rapidly evolving field which provides high contrast and high resolution 3-dimensional images of the heart, coronary vessels, and the great vessels without subjecting the patient to ionizing radiation. CMR is often considered a “one-stop shop” as it can provide a comprehensive assessment of the heart including myocardial wall motion, cavity size, ventricular ejection fraction, wall thickness, valvular function, infarct area, proximal coronary artery lesions, aortic disease including aneurysm and dissection, pericardial disease, congenital heart defects, and myocardial viability.
Myocardial viability:
Currently, the most important and common clinical use of CMR is assessing myocardial viability. Dysfunctional myocardium is deemed viable if it can regain function (contractility) after regional blood supply is improved via revascularization. Nonviable myocardium is dead or scar tissue which has permanently lost its function irrespective of its regional blood supply. Viability testing guides clinical practice in patients with left ventricular (LV) dysfunction as revascularization of nonviable myocardium would not improve myocardial contractility, ejection fraction, or mortality and may even increase morbidity and mortality by subjecting the patient to an unnecessary interventional procedure.
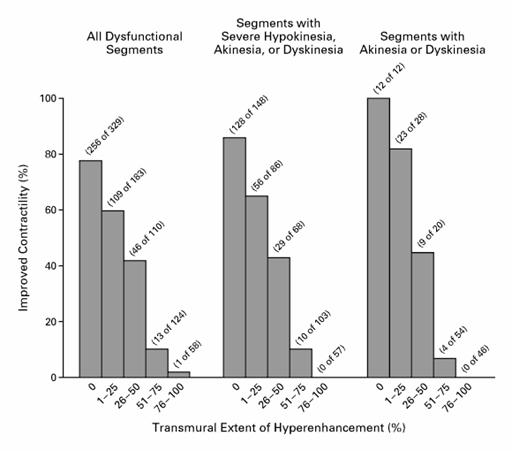
CMR is excellent at determining myocardial viability using late gadolinium enhancement (LGE or hyperenhancement). When gadolinium is “taken up” by nonviable or scarred myocardium, it is not washed out as quickly as healthy myocardium due to poor blood flow, and thus images taken > 10 minutes after gadolinium injection will exhibit hyperenhancement or late gadolinium enhancement (regions where gadolinium is retained). The transmural extent of LGE signifies the thickness of nonviable or scar tissue and this correlates well to areas that are less viable and are partially or completely damaged in an irreversible manner. Conversely, absence of LGE corresponds to areas of viable myocardium.(1,2,3 ) A study of 50 patients with ventricular dysfunction undergoing revascularization showed that CMR accurately identified viable myocardium before revascularization. Absence of late gadolinium enhancement correlated well with segments which improved function after revascularization (78% of segments without LGE had improved function). Presence of late gadolinium enhancement correlated accurately with segments which had no improvement after revascularization (less than 2% of segments with LGE had improved function). The probability of improvement in segmental function significantly decreased as the extent of transmural LGE (thickness of scar) increased (see Figure 1).(3 )
Figure 1: Relation between the Transmural Extent of Hyperenhancement before Revascularization and the Likelihood of Increased Contractility after Revascularization. 804 dysfunctional segments were identified by CMR in 50 patients who were scheduled to undergo revascularization. As the transmural extent of late gadolinium enhancement increased, the likelihood of post-revascularization improvement in contractility significantly decreased. (adapted from Kim et al.)(3)
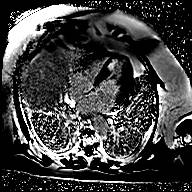
Figure 2: Cardiac MRI displaying late gadolinium enhancement
Traditionally, radionuclide myocardial perfusion imaging (using thallium, technetium-sestamibi, or positron emission tomography) and dobutamine echocardiography have been utilized to assess myocardial viability. CMR compares very favorably to these modalities and is even superior in some respects. In a study of 208 patients with suspected coronary artery disease referred for revascularization, dobutamine stress MRI had a 86% sensitivity and 74% specificity in terms of predicting viability when compared to dobutamine stress echocardiography which had a 85% sensitivity and 69% specificity.(4) When compared to positron emission tomography with F- 18 deoxyglucose (FDG-PET), CMR had similar sensitivity and specificity (94% and 84% respectively).(5) In comparison to resting thallium-201 perfusion imaging, CMR performs as well in terms of detecting transmural infarctions but is more accurate in detecting subendocardial infarctions.(6) Several studies have suggested that CMR may be more sensitive in detecting viability in regions of severely dysfunctional myocardium, which are often deemed non-viable by traditional techniques. CMR and conventional modalities are both poor at detecting viability in regions of intermediate residual viability but have similar performance in regions with high and low residual viability.
Clinical case applications:
Cardiac MRI can be used to assess for myocardial viability in our patient and thus guide future therapy (revascularization or optimal medical therapy). Furthermore, CMR can accurately determine ejection fraction and extent of proximal coronary artery atherosclerotic lesions, thus risk stratifying this patient. Left main or triple vessel disease may necessitate a surgical intervention in this patient. However, medical therapy or single vessel PCI may be more appropriate than CABG despite multivessel CAD if there is limited or no viability in multiple infarct areas.
In our patient, cardiac MRI/MRA showed mildly dilated LV with moderately reduced LV ejection fraction, hypokinetic myocardium without scar in LAD territory, hypokinetic myocardium with mild scar in RCA territory, and hypokinetic but viable myocardium in LCX territory. Coronary artery origins were incompletely visualized due to motion but there was patent origin of RCA, LAD, and LCX (L main couldn’t be visualized). Patient was discharged home on medical therapy and planned to return for coronary angiogram and possible revascularization as his coronary anatomy was not completely delineated by the Cardiac MRI.
REFERENCES
1. Kim, RJ, Fieno, DS, Parrish, TB, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 1999; 100:1992.
2. Fieno, DS, Kim, RJ, Chen, EL, et al. Contrast-enhanced magnetic resonance imaging of myocardium at risk: distinction between reversible and irreversible injury throughout infarct healing. J Am Coll Cardiol 2000; 36:1985.
3. Kim RJ, Wu E, Rafael A, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 2000; 343:1445-1453.
4. Nagel, E, Lehmkuhl, HB, Bocksch, W, et al. Noninvasive diagnosis of ischemia-induced wall motion abnormalities with the use of high-dose dobutamine stress MRI: comparison with dobutamine stress echocardiography. Circulation 1999; 99:763.
5. Kuhl HP, Beek AM, van der Weerdt AP, et al. Myocardial viability in chronic ischemic heart disease: comparison of contrast-enhanced magnetic resonance imaging with (18)F-fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol 2003;41:1341-8.
6. Bellenger NG, Burgess MI, Ray SG, et al. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance. Are they interchangeable? Eur Heart J 2000;21:1387-96.
Reviewed by Robert Donnino MD, NYU Division of Cardiology:
The case report and discussion provided by Dr. Ghumman highlights some of the important uses of CMR in patients with ischemic cardiomyopathies. In this case the CMR provided several important pieces of information relevant to this patient’s care. First, by the distribution of myocardial scar (seen as LGE on CMR) we can confirm with high confidence that this patient’s cardiomyopathy is ischemic in etiology. In addition, the presence of only mild scar (as opposed to transmural or near-transmural scar) indicates that this patient is very likely to have a significant improvement in left ventricular function after revascularization in all three major coronary vessel distributions. As mentioned, this assessment of viability using CMR has been proven to be very reliable and is being used with increasing frequency in patients undergoing coronary revascularization (particularly in those being considered for bypass surgery). While it was not possible in this patient due to motion artifact, CMR can also provide an assessment of coronary stenosis, particularly of the proximal and larger vessels. Although there is continuing progress, CMR remains somewhat inferior to CT angiography and invasive x-ray angiography for detection of coronary stenosis, particularly of distal arteries and smaller caliber vessels. Finally, CMR is considered the gold standard for measurements of left ventricular ejection fraction and chamber volume. This may further assist the clinician in determining therapies such as biventricular pacemakers or cardiac defibrillators, particularly in those patients with technically challenging echocardiograms.