By Michael Crist
Faculty Peer Reviewed
Until recently, little thought was given to the important role played by the duodenum, jejunum, and ileum in glucose homeostasis. The involvement of the gut in glucose regulation is mediated by the enteroinsular axis, which refers to the neural and hormonal signaling pathways that connect the gastrointestinal (GI) tract with pancreatic beta cells. These pathways are largely responsible for the increase in insulin that occurs during the postprandial period. In 1964 McIntyre and colleagues first reported the phenomenon of oral glucose administration eliciting a greater insulin response than a similar amount of glucose infused intravenously [1]. This observation, later named the incretin effect, accounts for the role of certain gut hormones within the enteroinsular axis that promote insulin secretion [2]. Although many hormones are believed to contribute, the two that play the most significant role in nutrient-stimulated insulin secretion are glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) [3,4]. GLP-1 is synthesized by L-cells found predominantly in the ileum and colon [5], and GIP is secreted from K-cells found predominantly in the duodenum [6]. Both GLP-1 and GIP are secreted in response to nutrients within the gut and are powerful insulin secretagogues, accounting for roughly 50% of postprandial insulin secretion [7]. They have both been shown to promote pancreatic beta cell proliferation and survival [7]. GLP-1 has also been shown to inhibit glucagon secretion and gastric emptying while promoting satiety and weight loss [7].
The incretin effect progressively diminishes with the onset of type 2 diabetes in a process that contributes to disordered glucose metabolism. GLP-1 secretion is significantly lower in type 2 diabetics than in non-diabetic individuals, and GIP loses its insulinotropic properties [8]. Malabsorptive bariatric surgery operations, which alter GI tract anatomy, have been shown to affect incretin hormone profiles and glucose homeostasis [9,10]. Many patients show a postoperative return to normal plasma glucose, plasma insulin, and glycosylated hemoglobin levels and discontinue the use of diabetes-related medication [10,11]. The dramatic resolution of diabetes and the return to euglycemia often occur within one week of surgery, before a significant amount of weight loss occurs [12,13]. In 2001 Pories and Albrecht reported long-term glycemic control in 91% of patients 14 years after they underwent malabsorptive bariatric surgery [12]. Furthermore, the improvement in insulin sensitivity postoperatively has been shown to prevent the progression from impaired glucose tolerance to diabetes [12].
The exact mechanism through which malabsorptive bariatric surgery improves glucose homeostasis is unclear. Much of the evidence in support of bariatric surgery as treatment for diabetes comes from studies that have focused on roux-en-Y gastric bypass and biliopancreatic diversion (which results in enteral nutrition passing directly from the stomach to the ileum) [9,10]. Both of these procedures surgically alter the GI tract such that nutrient chyme bypasses the duodenum and the proximal jejunum. Many initially hypothesized that enhanced nutrient delivery to the distal intestine promotes a physiological signal that ameliorates glucose metabolism. Enhanced GLP-1 secretion as a result of expedited nutrient delivery to the L-cell-rich ileum has been proposed as a mechanism that contributes to this process (14,15). Alternatively, the exclusion of nutrient flow through the duodenum and proximal jejunum may interrupt a signaling pathway that confers insulin resistance [11,12].
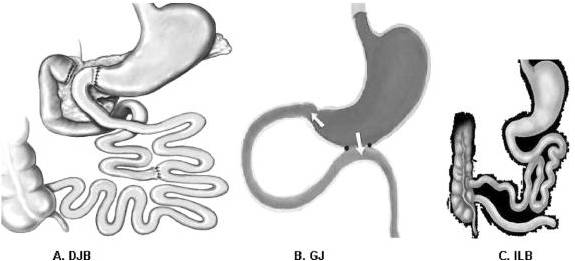
Rubino and colleagues tested both theories in a non-obese model of type 2 diabetic rats by comparing glucose tolerance among 3 surgery groups and one non-operated control. Of the 3 surgery groups, one underwent duodenal jejunal bypass (DJB), which excluded nutrient passage from the proximal foregut, resulting in early nutrient delivery to the distal gut. Another group underwent gastrojejunostomy (in which a surgical anastomosis was created between the stomach and jejunum while preserving the normal connection between the stomach and duodenum). This allowed both the normal passage of nutrients through the foregut and enhanced nutrient delivery to the hindgut. In effect, both the DJB and gastrojejunostomy promoted nutrient delivery to the ileum, whereas only the DJB procedure excluded the duodenum from nutrient passage. The third group was a sham-operated control. The DJB group showed better glucose tolerance than all other study groups even though there were no differences in food intake, body weight, or nutrient absorption [16]. Furthermore, when the DJB group underwent a second operation to allow nutrient passage through the foregut, glucose tolerance deteriorated. When the gastrojejunostomy group underwent a second operation to prevent nutrient passage through the foregut, glucose tolerance improved [16]. These findings suggest that exclusion of the duodenum and proximal jejunum is a necessary component of surgical interventions aimed at improving glucose tolerance.
Bariatric surgery holds great potential in the treatment of T2DM and will likely play an increasingly important role in diabetes management. Improved glucose regulation following malabsorptive bariatric surgery procedures is likely multifactorial, with alterations in gut microflora and the beneficial effects of weight loss contributing with time. Changes in gut hormone secretion profiles, however, appear to play an important role in the initial improvements reported in glucose homeostasis.
FIGURE 1. Interventions. A, Duodenal-jejunal bypass (DJB). This operation does not impose any restriction to the flow of food through the gastrointestinal tract. The proximal small intestine is excluded from the transit of nutrients, which are rapidly delivered more distally in the small bowel. Food exits the stomach and enters the small bowel at 10 cm from the ligament of Treitz, and digestive continuity is reestablished approximately 25% of the way down the jejunum. B, Gastrojejunostomy (GJ). This operation consists of a simple anastomosis between the distal stomach and the first quarter of the jejunum. The site of the jejunum that is anastomosed to the stomach is chosen at the same distance as in DJB (10 cm from the ligament of Treitz). Hence, the DJB and GJ share the feature of enabling early delivery of nutrients to the same level of small bowel. In contrast to DJB, the GJ does not involve exclusion of duodenal passage, and nutrient stimulation of the duodenum is maintained. C, Ileal bypass (ILB). This operation reduces intestinal fat absorption by preventing nutrients from passing through the distal ileum, where most lipids are absorbed.
Michael Crist is a 4th year medical student at NYU School of Medicine
Peer reviewed by Natalie Levy, MD, Department of Medicine (GIM Div.) NYU Langone Medical Center
References
1. McIntyre N, Holdsworth CD, Turner DS. New interpretation of oral glucose tolerance. Lancet. 1964;2(7349):20-21.
2. Creutzfeldt W. The incretin concept today. Diabetologia. 1979;16(2):75-85. http://www.ncbi.nlm.nih.gov/pubmed/32119
3. Fetner R, McGinty J, Russell C, Pi-Sunyer FX, Laferrère B. Incretins, diabetes, and bariatric surgery: a review. Surg Obes Relat Dis. 2005;1(6):589-597.
4. Drucker DJ. Enhancing incretin action for the treatment of type 2 diabetes. Diabetes Care. 2003;26(10):2929-2940. http://care.diabetesjournals.org/content/26/10/2929
5. Drucker DJ. Incretin-based therapies: A clinical need filled by unique metabolic effects. Diabetes Educ. 2006;32 Suppl 2:65S-71S.
6. Vilsbøll T, Holst JJ. Incretins, insulin secretion and type 2 diabetes mellitus. Diabetologia. 2004;47(3):357-366.
7. Fetner R, McGinty J, Russell C, Pi-Sunyer FX, Laferrère B. Incretins, diabetes, and bariatric surgery: a review. Surg Obes Rel Dis. 2005;1(6):589-597.
8. Nauck M, Stöckmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986;29(1):46-52. http://www.ncbi.nlm.nih.gov/pubmed/3514343
9. Rosa G, Mingrone G, Manco M, et al. Molecular mechanisms of diabetes reversibility after bariatric surgery. Int J Obes (Lond). 2007;31(9):1429-1436. http://www.ncbi.nlm.nih.gov/pubmed/17515913
10. Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238(4):467-84.
11. Rubino F, Gagner M, Gentileschi P, et al. The early effect of the Roux-en Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240(2):236-242.
12. Pories WJ, Albrecht RJ. Etiology of type II diabetes mellitus: role of the foregut. World J Surg. 2001;25(4):527-531. http://www.ncbi.nlm.nih.gov/pubmed/11344408
13. Guidone C, Manco M, Valera-Mora E, et al. Mechanisms of recovery from type 2 diabetes after malabsorptive bariatric surgery. Diabetes. 2006;55(7):2025-2031.
14. Patriti A, Aisa MC, Annetti C, et al. How the hindgut can cure type 2 diabetes. Ileal transposition improves glucose metabolism and beta-cell function in Goto-kakizaki rats through an enhanced Proglucagon gene expression and L-cell number. Surgery. 2007;142(1):74-85. https://www.ncbi.nlm.nih.gov/m/pubmed/17630003/?i=2&from=/16259883/related
15. Patriti A, Facchiano E, Sanna A, Gulla N, Donini A. The enteroinsular axis and the recovery from type 2 diabetes after bariatric surgery. Obes Surg. 2004;14(6):840-848.
16. Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244(5):741-749.