Peer Reviewed
As many of us reflect on the festivities that occurred during the Department of Medicine Holiday Party this past weekend, all can agree that winter is not only coming, but that it has arrived. Earlier this week those inside New York University’s Langone Medical Center Tisch Hospital may have thought the excessive heating was premature, only to realize that a fire had broken out on 7th floor of the currently-under-construction Kimmel Pavilion. No injuries were reported, and the two-alarm fire was quickly put out in less than an hour by 8 engine trucks, 5 ladder trucks, and over 100 firefighters at the scene.[2-3] While most would have preferred less energy at work, there was no shortage of it within the President-elect Trump Administration, as he announced ex-Governor of Texas Rick Perry as his nominee for Secretary of Energy.[4] During the 2012 republican presidential primary debate, Mr. Perry famously forgot the Department of Energy as one of three government agencies he would cut if elected president.
Passive Leg Raising for Predicting Fluid Responsiveness: a Systematic Review and Meta-Analysis[5]
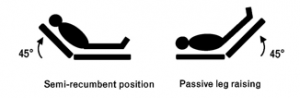
While many feel that there is obvious uneven thinking in the head-elect of our country, Monnet et al. conducted a systematic review and meta-analysis, published in Intensive Care Medicine, about fluid responsiveness to a passive leg raise. The procedure of the passive leg raise varied throughout the 21 studies reviewed, but generally a patient is placed in the semi-recumbent position at 45 degrees for 3 minutes, followed by placing the patient supine and then passively raising the legs to 45 degrees (figure 1).
Figure 1: Patients are placed in the semi-recumbent position at 45 degrees for 3 minutes, followed by placing the patient supine and then passively raising the legs to 45 degrees. This can simulate a 300cc fluid bolus.[6]
In the absence of increased intra-abdominal pressure or pathology, this increases the venous return to the heart by about 300cc for 10-30 seconds.[7] In the case of preload dependence of both ventricles, the resultant virtual 300cc fluid bolus then increases the patient’s cardiac output. The authors’ review of MEDLINE, EMBASE, and Cochrane from 1966 to 2015 was limited to articles reporting sensitivity and specificity of the passive leg raise test to predict fluid responsiveness when assessing its effect on cardiac output and arterial pressure. Twenty-one studies were selected, totaling 991 adult patients and 995 fluid challenges. Fluid responsiveness was determined by an increase in cardiac output (or surrogate defined as cardiac index, stroke volume, aortic flow velocity, aortic velocity time integral) by more than 7-15% (16/21 studies used 15%). On average the increase in cardiac output was 20 ± 9%, while the pooled sensitivity was 85% and pooled specificity was 91%. While the passive leg raise has never been demonstrated to improve survival, it has been recommended by a recent consensus conference of the European Society of Intensive Care Medicine and implemented in the bundles of the surviving sepsis campaign.[8-9]
Effect of CPAP Withdrawal on BP in OSA: Data from Three Randomized Controlled Trials[10]
Ever feel that interviewing a patient through a CPAP device is like having a conversation with Darth Vader? Ever want to take the mask off the patient so you can get a real history? While Doc Vader reflects on a productive year before the New Year[11], a study by Schwarz et al., published in Chest, analyzed data from three randomized controlled trials about the effect of continuous positive airway pressure (CPAP) withdrawal on blood pressure in obstructive sleep apnea (OSA).[12-13] OSA is a sleep-related breathing disorder defined by repetitive oxygen desaturation during sleep. The suggested pathophysiological mechanism of hypertension in OSA is best thought to include increased stress from intermittent hypoxia with increased sympathetic activity and endothelial dysfunction. The increased intrathoracic pressures also cause increased transmural pressure on the heart and local blood vessels, triggering long term cardiovascular remodeling.[14-17] The most effective treatment for OSA is CPAP, which prevents pharyngeal collapse during sleep and has been shown to lower systolic and diastolic blood pressure by 2.5 mm Hg and 2 mm Hg, respectively. Schwarz et al. analyzed data representing 153 patients with established moderate to severe OSA who were receiving CPAP therapy. This patient group was identified because they were familiar to the authors and they all participated in three similar trials with similar designs. This patient cohort was subsequently randomized to either continue CPAP therapy or discontinue it for two weeks. CPAP withdrawal was found to increase systolic blood pressure by 5.4 mm Hg or 9 mm Hg when measured in a physician office versus patient self-reported monitoring, respectively. Diastolic blood pressure was similarly raised 5 mm Hg and 7.8 mm Hg for physician office versus self-reported home measurements, respectively. Interestingly, CPAP withdrawal in this study resulted in a 20.2% increase in defined hypertension (systolic BP > 140 or diastolic BP > 90) in previously normotensive patients. This suggests that CPAP hardware breakdowns can produce moderate increases in blood pressure in patients that were previously controlled, and should be replaced promptly to prevent rebound uncontrolled hypertension. Unfortunately the study does not comment on resulting symptoms or other outcomes.
As daytime somnolence is a constant struggle in the hospital, sometimes we wish Starbucks would drop a red or black eye into our coffee. Caffeine, a methylxanthine compound related to theophylline, is the most widely consumed sympathetic nervous system stimulant in the world. Previously in rabbit [19] and dog [20] models, caffeine infusions were associated with ventricular premature beats and ventricular arrhythmias, respectively. In addition, two case reports have described fatal caffeine ingestions in humans [21-22]. This helped to establish a now controversial presumption that excessive caffeine could lead to severe arrhythmias. In a double-blinded randomized clinical trial published in JAMA Internal Medicine, Zuchinali et al. sought to compare the effect of high-dose caffeine or placebo on the frequency of supraventricular and ventricular arrhythmias both at rest and during a treadmill stress test (Naughton protocol) in patients with chronic heart failure and an ejection fraction < 45% (etiology was generally non-ischemic) and New York Heart Association functional class I to III symptoms (most patients were I or II). Patients were also receiving standard-of-care heart failure drug therapy, but were excluded if they were taking any antiarrhythmic drugs besides β-blockers and amiodarone. Approximately 50% of the 51 enrolled patients were habitual coffee drinkers, however all patients were asked to self-restrict their caffeine intake one week prior to the study. Patients were then connected to a continuous EKG machine and randomized to ingest 5 doses of 100mL decaffeinated coffee mixed with 100mg of either caffeine or lactose powder at 1-hour intervals for 5 hours. Overall, no significant differences in ventricular premature beats or supraventricular premature beats (isolated, couplets, or non-sustained tachycardia) were observed between groups at rest (6.7 hours of EKG monitoring) or during the treadmill test. These results challenge the notion that caffeine intake should be limited or prohibited in patients with heart disease at risk for arrhythmia.
Significant differences have been described between women and men regarding presentation, mechanism, and treatment outcomes of ventricular tachycardia (VT). The authors of a multicenter, observational study published in JAMA Cardiology looked at 12 high-volume ablation centers studying consecutive patients with structural heart disease (left ventricular ejection fraction < 55%, hypertrophic cardiomyopathy, or right ventricular cardiomyopathy, each with scar confirmed during electroanatomic mapping) undergoing ventricular tachycardia ablation from 2002-2013. Prior studies have noted gender differences among different cardiac arrhythmias, however studies of VT ablation have not included sufficient numbers of women (rarely more than 10% of study population) to determine if significant differences exist in terms of clinical presentation, electrophysiologic substrate, and outcome. Of the 2062 patients that underwent VT ablation in the JAMA study, 12.9% were women. All patients underwent intra-procedure endocardial mapping, and while the ablation strategy varied from center to center, emphasis was placed on eliminating clinical VT(s). Other forms of VT were targeted, including nonclinical VT. The primary endpoint was VT recurrence (defined as 30+ seconds of sustained VT or VT requiring ICD treatment) following the last ablation procedure. Secondary outcomes included death and heart transplant. Compared with men, women were typically younger, had higher left ventricular ejection fractions, less VT storm, and lower prevalence of medical comorbidities including atrial fibrillation, diabetes, and hypertension. While the number of induced VT episodes was similar between men and women, women had shorter ablation time and were more likely to have clinical VT inducible at the end of the ablation. After the procedure, women not only had a shorter time until recurrence, but they also had more recurrence. The amount and timing for recurrence was most pronounced between genders when looking at ischemic cardiomyopathies. Despite having more favorable baseline characteristics, women had worse VT-free survival following ablation. The authors suggest four explanations; (1) women may undergo ablation at later stages of disease (rebutted by authors), (2) VT ablation is more challenging in women for reasons not yet understood, (3) women are undertreated during ablation, (4) women are undertreated following ablation. This is the first study enroll a significant number of women and to document statistically significant gender discrepancies with regards to VT ablation.
Also in the news,
In a retrospective cohort study in 4 affiliated but geographically distinct hospitals in the New York City Metropolitan area from 2010-2015, antibiotic receipt by prior bed occupants was associated with increased risk of Clostridium difficile infection in subsequent patients.[24]
The CDC’s annual Youth Risk Behavior Survey reports a compelling view into the different experiences self-reported by lesbian, gay, bisexual, not sure, and straight adolescents. Importantly student self-reporting of sexual identity, same-sex sexual contact, and students who are unsure of their sexual identity, occur during adolescence but this process may not be necessarily linear. While these populations remain significantly vulnerable to bullying, maladaptive risk behaviors such as drug use could represent an attempt to cope with associated prejudice and stigma.[25]
A quantitative direct observational and self-reported study of physicians in various medicine practices (family medicine, internal medicine, cardiology, orthopedics) in an ambulatory care setting were monitored with regards to how their time was spent. Overall, direct clinical face time with patients occupied 27% of the office day, while 49.2% was spent on the electronic health record and desk work. When only analyzing time in the examination room with patients, physicians spent 52.9% of the time on direct clinical face time and 37% on electronic health record work.[26]
Among countries ranked by total GDP in 2015, the United States had 10 times more deaths by firearm assaults than the next 4 highest countries by GDP combined (China, Japan, Germany, and the United Kingdom).[27]
B. Corbett Walsh, MD, is a second year medical resident at NYU Langone Medical Center
Peer Reviewed by Jennifer Mulliken, MD, Senior Chief Resident in Internal Medicine at NYU Langone Medical Center and Associate Editor of Clinical Correlations.
References:
[1] https://twitter.com/NYCityAlerts/status/809086258643369985/photo/1
[2] https://www.facebook.com/NYULangone/posts/10154813704688703
[3] http://www.fdnewyork.com/aa.asp
[4] http://www.nytimes.com/2016/12/13/us/politics/rick-perry-energy-secretary-trump.html
[5] Monnet X1,2, Marik P3, Teboul JL. Passive leg raising for predicting fluid responsiveness: a systematic review and meta-analysis. Monnet X1,2, Marik P3, Teboul JL. Intensive Care Med. 2016 Dec;42(12):1935-1947.
[6] https://i1.wp.com/emcrit.org/wp-content/uploads/2011/12/plr-bw.png
[7] Jabot J, Teboul JL, Richard C, Monnet X: Passive leg raising for predicting fluid responsiveness: importance of the postural change. Intensive Care Med 2009, 35:85–90.
[8] Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, Jaeschke R, Mebazaa A, Pinsky MR, Teboul JL, Vincent JL, Rhodes A (2014) Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med 40:1795–1815
[9] Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R (2015) Updated bundles in response to new evidence. In: Surviving sepsis campaign. Crit Care Med. 2013 Feb;41(2):580-637.
[10] Schwarz EI, Schlatzer C, Rossi VA, Stradling JR, Kohler M. Effect of CPAP Withdrawal on BP in OSA: Data from Three Randomized Controlled Trials. Chest. 2016 Dec;150(6):1202-1210.
[11] https://www.youtube.com/user/ZDoggMD/videos
[12] kohler M, Stoewhas AC, Ayers L, et al. Effects of continuous positive airway pressure therapy withdrawal in patients with obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2011;184(10):1192-1199. 26.
[13] Rossi VA, Winter B, Rahman NM, et al. The effects of Provent on moderate to severe obstructive sleep apnoea during continuous positive airway pressure therapy withdrawal: a randomised controlled trial. Thorax. 2013;68(9): 854-859. 27.
[14] Schwarz EI, Schlatzer C, Stehli J, et al. Effect of CPAP withdrawal on myocardial perfusion in OSA: a randomized controlled trial. Respirology. 2016;21: 1126-1133.
[15] Schwarz EI1, Schlatzer C1, Rossi VA1, Stradling JR2, Kohler M3. Effect of CPAP Withdrawal on BP in OSA: Data from Three Randomized Controlled Trials. 2016 Dec;150(6):1202-1210.
[15] Kohler M, Stradling JR. Mechanisms of vascular damage in obstructive sleep apnea. Nat Rev Cardiol. 2010;7(12):677-685.
[16] Kohler M, Stradling JR. CrossTalk proposal: Most of the cardiovascular consequences of OSA are due to increased sympathetic activity. J Physiol. 2012;590(12):2813-2815; discussion 2823.
[17] Narkiewicz K, Somers VK. Sympathetic nerve activity in obstructive sleep apnoea. Acta Phys Scand. 2003;177(3): 385-390.
[18] Zuchinali P, Souza GC, Pimentel M, Chemello D, Zimerman A, Giaretta V, Salamoni J, Fracasso B, Zimerman LI, Rohde LE. Short-term Effects of High-Dose Caffeine on Cardiac Arrhythmias in Patients With Heart Failure: A Randomized Clinical Trial. JAMA Intern Med. 2016 Dec 1;176(12):1752-1759.
[19] Ishida S, Ito M, Takahashi N, Fujino T, Akimitsu T, Saikawa T. Caffeine induces ventricular tachyarrhythmias possibly due to triggered activity in rabbits in vivo.Jpn Circ J. 1996;60(3):157-165
[20] Mehta A, Jain AC, Mehta MC, Billie M. Caffeine and cardiac arrhythmias: an experimental study in dogs with review of literature. Acta Cardiol. 1997;52 (3):273-283.
[21] Jabbar SB, Hanly MG. Fatal caffeine overdose: a case report and review of literature. Am J Forensic Med Pathol. 2013;34(4):321-324.
[22] Kerrigan S, Lindsey T. Fatal caffeine overdose: two case reports. Forensic Sci Int. 2005;153(1):67-69.
[23] Frankel D, Tung R, Santangeli P, Tzou WS, Vaseghi M, Di Biase L, Nagashima K, Tedrow U, Bunch TJ, Tholakanahalli VN, Dendi R, Reddy M, Lakkireddy D, Dickfeld T, Weiss J, Mathuria N, Vergara P, Patel M, Nakahara S, Vakil K, Sauer WH, Callans DJ, Natale A, Stevenson WG, Della Bella P, Shivkumar K, Marchlinski FE. Sex and Catheter Ablation for Ventricular Tachycardia: An International Ventricular Tachycardia Ablation Center Collaborative Group Study. JAMA Cardiol. 2016 Nov 1;1(8):938-944.
[24] Freedberg DE, Salmasian H, Cohen B, Abrams JA, Larson EL. Receipt of Antibiotics in Hospitalized Patients and Risk for Clostridium difficile Infection in Subsequent Patients Who Occupy the Same Bed. JAMA Intern Med. 2016 Dec 1;176(12):1801-1808.