 By Carolyn B. Drake, MD, MPH
By Carolyn B. Drake, MD, MPH
Peer Reviewed
News:
In the fortnight since Donald Trump was inaugurated as the 45th president of the United States, we’ve seen a flurry of political activity as the new president seeks to …

 By Carolyn B. Drake, MD, MPH
By Carolyn B. Drake, MD, MPH
Peer Reviewed
News:
In the fortnight since Donald Trump was inaugurated as the 45th president of the United States, we’ve seen a flurry of political activity as the new president seeks to …
 By Samantha K. Newman, MD
By Samantha K. Newman, MD
Peer Reviewed
As Melania and Barron Trump are (not) settling into the White House, our new president spent Friday afternoon issuing an executive order that bans individuals from seven …
By Calvin Ngai, MD
Peer Reviewed
In former President Barack Obama’s last farewell speech, he asked all fellow Americans to continue to believe in our ability to create change. This past weekend, the day after Donald J. Trump was sworn into the presidential office and our country bid one last farewell to …
 By Scott Butler, MD
By Scott Butler, MD
Peer Reviewed
A presidential goodbye. A contentious press conference. A salacious dossier from a British spy. It’s been quite a week.
As Republican legislators transition from symbolically voting to …
 By: Nancyanne Schmidt, MD
By: Nancyanne Schmidt, MD
Peer Reviewed
This week, the intelligence report commissioned by President Obama on suspected hacking during the recent presidential election revealed that Russian President Vladimir Putin ordered an influence campaign …
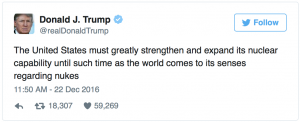 By Derek Moriyama, MD
By Derek Moriyama, MD
Peer Reviewed
It is official. The members of the electoral college have cast their votes and Donald Trump will be the next President of the United States. As he …
 By B. Corbett Walsh, MD
By B. Corbett Walsh, MD
Peer Reviewed
As many of us reflect on the festivities that occurred during the Department of Medicine Holiday Party this past weekend, all can agree that winter is not only coming, but that it has arrived. Earlier this week …
 By Shriram Alapaty, MD
By Shriram Alapaty, MD
Peer Reviewed
This week, America’s president-elect Donald Trump continued to defy expectations by selecting Mr. Scott Pruitt, a close ally of the fossil fuel industry, to head the Environmental Protection …