Faculty Peer Reviewed
Since the start of vaccination – we’ve eradicated smallpox and polio, saved college kids from meningitis, averted flu epidemics, and decreased the incidence of HPV-related cervical cancer … but can we teach our immune systems to actively fight existing cancer?
Here’s the mechanism for an ideal anti-cancer vaccine:
With the growth and turnover of cancerous cells, cancer-specific tumor-associated antigens (TAAs) would be recognized and processed by professional antigen-presenting cells (APCs), such as dendritic cells and macrophages – which would present the antigens to T-cell receptors (TCR) via unique MHC:TCR binding sites. Intracellular antigens would be presented via the MHC class I molecules directly to cytotoxic CD8 cells, whereas extracellular antigens on cell-membranes would be presented via the MHC class II molecules to Cd4 helper cells. Despite this general rule, there also exists the possibility of cross-presentation by a minority of the dendritic cell subtypes – by which extracellular antigens can be presented to CD 8 cells via MHC-I (and vice versa). For example, as rapidly proliferating cancer cells undergo breakdown (autophagy), cell membrane components (that would normally be presented to CD4 cells via MHC-II) are engulfed by phagosomes and subsequently fused with and processed by liposomes – thereby allowing these extracellular cytoplasmic components to be presented to CD8 cells as well. Meanwhile, the cytotoxic CD8 response would be enhanced by the supporting CD4 helper response.
Here’s the reality:
Cancers are indeed “infiltrated with dendritic cells early in the course of disease – approximately 30% of node-negative early stage breast cancers have significant dendritic cell infiltrationâ€. [1] Immune anti-tumor response may even be a prognostic indicator – for example, in ER-/HER2- breast cancer, each 10% increase in intratumor lymphocytic infiltration correlates with a 17% risk reduction of relapse and a 27% risk reduction of death [2]. The problem, however, is that the majority of tumor-associated antigens are actually over-expressed products of normal cellular genes. This means, that as a result of negative thymic selection early in T-cell development, only a mild-moderate affinity MHC:TCR binding occurs, and thus a less than ideal cytotoxic T-cell response. [3] Finally, most of the tumor-associated antigens are of intracellular protein origin and thus presented via the MHC Class I to CD8 T-cells only – resulting in a brief cytotoxic response with little CD4 enhancement/antibody response and only a moderate affinity CD-8 response [1]. To complicate matters further, while the Th1 Cd4 cells augment the CD8 and macrophage response via cytokines like IL-2 and TNF-alpha, the Th2 CD4 cells are less helpful, and may even be detrimental in cancer immunotherapy. Th2 CD4 cells produce IL4 cytokine (which functions as a regulator of B-cell expansion and therefore enhances cancer cell survival) as well as IL13, less well understood but known to correlate with metastatic tumor spread. [4] In the world of cancer vaccine, therefore, there is a focus on enhancing the CD8 and Th1 CD4 response while down-regulating the Th2 CD4 response – but more on this later.
The making of a cancer vaccine:
There are different approaches to tumor antigen delivery. Tumor antigens can be whole tumor cells (i.e: irradiated/lysed cancer cells from an autologous or allogenic source such as a previously resected tumor) or just parts of a cell (full length proteins). Alternatively, antigens can be specific peptides, which in turn can be loaded onto dendritic cells (DCs) in-vivo using chimeric proteins made of anti-DC receptor Ab; and subsequently re-infused into the patient. Finally, antigens can also be DNA or RNA strands that are transduced or transfected in a vector. For a more rigorous immune response to a vaccine, antigens can be combined with adjuvants, or boosters known to augment immune system activation (common examples include general immune stimulants like the BCG vaccine or non-specific bacterial products, as well as specific immune system activators such as the GCF stimulating factors). [5, 6]
Peptide vaccines are the most common, and one such example is the gp100 peptide from a melanoma antigen. In 2011, a phase 3 randomized control trial compared the standard treatment for advanced melanoma at the time, interleukin-2 (IL-2), with a vaccine (primed with gp-100 peptide) plus IL-2. [7] In the end, complete response was seen in 9% vs 1% (p-value 0.02) in the vaccine vs control group respectively. Although the benefit was minor, the interesting finding was that on immunologic analysis, anti-peptide reactivity developed in none of the 12 control patients as compared with 7 of the 37 tested patients in the experimental group. Why did only 7 of these 37 patients show the immunologic response?
Several factors play a role in inducing, and maintaining, an immunological response. First, a strong cytotoxic T-cell response must be induced. This is limited by self-immunity and the difficulty in identifying an effective antigen. Furthermore, even if good activation of cytotoxic CD8 T-cells is achieved – this activity may not be enough for a sustained long-term response without the support of helper/memory T-cells. Animal studies have shown that CD-8 activation via MHC-I presented antigens did induce a direct antitumor effect, however these same CD-8 cells were unable to support themselves in the absence of helper activity from CD4 T-h cells, which in turn are activated via the MHC-II antigens. [1] This brings us to the second point, that induction of a CD4 Th1 helper response is important in maintaining anti-tumor CD8 T-cell responsiveness. Third, regulatory T-cell response (such as the IL-4 and IL-13 producing Th2 cells) must be eliminated/suppressed. The failure of the gp-100 peptide to induce and maintain a good cytotoxic response may therefore stem from the failure to induce direct CD8 activation vs. failure to boost the cytotoxic response with a helper CD4 response vs. interference of response by regulatory cells/ctyotkines.
So, what do we have so far that works?
An alternative approach by Kantoff et al resulted in the first FDA approved anti-cancer vaccine known as Sipuleucel-T, approved for castration resistant, metastatic prostate cancer. [8] The vaccine consists of blood cells collected from individual patients (leukophoresis) and then fused with a protein called PA2024, which is a combination of a prostate antigen called prostatic acid phosphotase, and a granulocyte-macrophage-colony-stimulating factor and re-infused. The phase 3 trial involved 512 men with metastatic, castration resistant prostate cancer and showed a relative risk reduction for mortality of 22% (P=0.03). However, the validity of the trial was subsequently questioned. [9] Specifically, it was noted that patients aged >65y/o in the placebo arm had a higher than predicted mortality and that it was this age group that contributed to the significantly improved mortality in the vaccine arm of the trial; whereas patients <65y/o did not significantly benefit from the vaccine. It was therefore suggested that while leukophoresis removed the majority of mononuclear cells, only the control group received GM-CSF as part of the vaccine – and that the ultimate difference in mortality may be driven by an induced “immunodeficient†state in the control patients rather than a benefit from the vaccine itself. In support of this latter theory is the discomforting finding that at the end of the experiment, T-cell and B-cell reactivity against the original PA2024 antigen was only 28% and 27% respectively – suggesting a non-specific stimulating effect of GMCF with little selection for the original peptide antigen.
A similar concept of activating autologous immune cells against cancer is that of adoptive transfer of tumor-infiltrating lymphocytes (TIL). Simply put, autologous TILs are obtained from the patient’s tumor, expanded in vitro and subsequently re-infused into the patient after a course of lymphodepleting chemotherapy. In metastatic melanoma, response rates range from 40-50% with a small subpopulation of complete remission – although overall 3-year survival in those who do not achieve complete remision still remains low at 36%. [10, 11] An alternative to TIL therapy are the genetically engineered lymphocytes selected against a specific tumor antigen. Early phase I trials in metastatic colon ca and melanoma have shown clinical benefit, but have also raised concerns for significant side-effects when normal tissue is attacked. [12]
Approaching cancer immunotherapy from a different angle are attempts to eliminate the inhibitory T-cell checkpoints thereby allowing for a more potent, less regulated, cytotoxic t-cell response. Examples of such regulatory molecules are the CTLA-4 (cytotoxic t-lymphocyte associated antigen 4) and PD1 (programmed death 1) – both are inhibitory receptors expressed on T-cells and involved in downregulating T-cell activation. [13] In 2010, a monoclonal antibody blocking CTLA-4 (ipilimumab) was shown to improve survival by four months in patients with unresectable stage III or IV melanoma. [14]. More recently, in July 2013, two phase 1 clinical trials showed that monotherapy with anti- PD-1 receptor as well as combination therapy with anti-CTLA-4 and anti-PD-1 can induce an objective response in up to 50% of patients. [15, 16]
Summary:
The concept of giving our immune system a boost in the already innate ability to recognize and destroy cancer cells is ambitious and fraught with difficulty, but perhaps not impossible. Current ongoing research to improve current immune therapies ranges on the one hand, from molecular activation of immune cell receptors (e.g: CD40) mediating an overall pro-inflammatory response [17] to, on the other hand, using ionizing radiation to induce a pro-inflammatory cell injury response as a booster for antigen recognition and immune system activation [18]. So although we cannot yet use vaccines to eliminate cancer like we did smallpox and polio, we perhaps are – one step at a time- making advances in that direction.
Dr. Jenny Gartshteyn is a 3rd year resident at NYU Langone Medical Center
Peer reviewed by Sylvia Adams, Associate Professor, Medicine, NYU Langone Medical Center
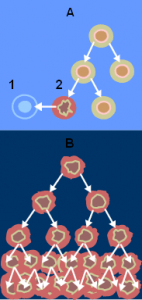
Image courtesy of Wikimedia Commons (A – normal cell division, B – cancer cell division; 1 – apoptosis; 2 – damaged cell. From the National Cancer Institute)
References:
1. Knutson, K.L. and M.L. Disis, Augmenting T helper cell immunity in cancer. Curr Drug Targets Immune Endocr Metabol Disord, 2005. 5(4): p. 365-71. http://www.ncbi.nlm.nih.gov/pubmed/16375690
2. Loi, S., et al., Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol, 2013. 31(7): p. 860-7. http://www.ncbi.nlm.nih.gov/pubmed/23341518
3. Durrant, L.G. and J.M. Ramage, Development of cancer vaccines to activate cytotoxic T lymphocytes. Expert Opin Biol Ther, 2005. 5(4): p. 555-63. http://www.ncbi.nlm.nih.gov/pubmed/15934833
4. Hallett, M.A., K.T. Venmar, and B. Fingleton, Cytokine stimulation of epithelial cancer cells: the similar and divergent functions of IL-4 and IL-13. Cancer Res, 2012. 72(24): p. 6338-43. http://www.ncbi.nlm.nih.gov/pubmed/23222300
5. Renno, T., et al., What’s new in the field of cancer vaccines? Cell Mol Life Sci, 2003. 60(7): p. 1296-310.
6. Palucka, K., H. Ueno, and J. Banchereau, Recent developments in cancer vaccines. J Immunol, 2011. 186(3): p. 1325-31. http://www.ncbi.nlm.nih.gov/pubmed/21248270
7. Schwartzentruber, D.J., et al., gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med, 2011. 364(22): p. 2119-27. http://www.ncbi.nlm.nih.gov/pubmed/21631324
8. Kantoff, P.W., et al., Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med, 2010. 363(5): p. 411-22. http://www.ncbi.nlm.nih.gov/pubmed/20818862
9. Huber, M.L., et al., Interdisciplinary critique of sipuleucel-T as immunotherapy in castration-resistant prostate cancer. J Natl Cancer Inst, 2012. 104(4): p. 273-9. Â http://jnci.oxfordjournals.org/content/early/2012/01/09/jnci.djr514.full
10. Besser, M.J., et al., Adoptive Transfer of Tumor Infiltrating Lymphocytes in Metastatic Melanoma Patients: Intent-to-Treat Analysis and Efficacy after Failure to Prior Immunotherapies. Clin Cancer Res, 2013.
11. Rosenberg, S.A., et al., Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res, 2011. 17(13): p. 4550-7. http://www.ncbi.nlm.nih.gov/pubmed/21498393
12. Park, T.S., S.A. Rosenberg, and R.A. Morgan, Treating cancer with genetically engineered T cells. Trends Biotechnol, 2011. 29(11): p. 550-7. http://www.ncbi.nlm.nih.gov/pubmed/21663987
13. Pardoll, D.M., The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer, 2012. 12(4): p. 252-64. http://www.ncbi.nlm.nih.gov/pubmed/22437870
14. Hodi, F.S., et al., Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med, 2010. 363(8): p. 711-23.
15. Hamid, O., et al., Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med, 2013. 369(2): p. 134-44.
16. Wolchok, J.D., et al., Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med, 2013. 369(2): p. 122-33.
17. Zhang, B., et al., The CD40/CD40L system: A new therapeutic target for disease. Immunol Lett, 2013. 153(1-2): p. 58-61. http://scibite.com/site/library/2013_7/1/0/23892087.html
18. Formenti, S.C. and S. Demaria, Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst, 2013. 105(4): p. 256-65.