 Commentary by Fritz Francois, MD, MS, NYU Division of Gastroenterology
Commentary by Fritz Francois, MD, MS, NYU Division of Gastroenterology
Humans are essentially the only reservoir for Helicobacter pylori, which is estimated to colonize the stomach of about half the world’s population (1). Although the bacteria generally do not invade the mucosa, attachment to the epithelium leads to an inflammatory reaction with neutrophils, lymphocytes, plasma cells, and macrophages. Over time, the persistent inflammation leads to changes in the gastric mucosa that may predispose to the development of dysplasia(2).
The known costs of H. pylori colonization include increased risk of peptic ulcer disease (PUD)(3), gastric adenocarcinoma (4), and gastric lymphoma (5). In epidemiological studies, colonization with H. pylori has been demonstrated to be associated with an increased risk of non-cardia gastric adenocarcinoma, especially in individuals who have harbored the organism for 10 years or longer (6). It is also well established that elimination of H. pylori changes the natural history of PUD (7) and gastric MALT-type lymphoma (8). Over the past 20 years, regimens that use acid-suppressing agents, combined with multiple antibiotics, and in particular clarithromycin have been highly successful for H. pylori eradication.
There have been well over 1300 H. pylori eradication trials with more than 140 different medication regimens(9). These trials have varied not only in the number and type of agents used but also in the duration of therapy. A first glance the literature seems to suggest (and many practitioners believe) that an eradication rate above 90% can consistently be achieved. Therefore it would seem that confirming eradication may not be necessary since most individuals will be successfully treated. However, a closer look at the eradication studies reveals some interesting points:
Many of the trials were quite small with a median of 30 individuals per trial (IQR 19-20)
The per protocol cure rate was only 71% (IQR 44-85%)
Taking into account those who could not complete the regimens, the per intention to treat cure rate was 67% (IQR 42-82%)
Therefore, we are not as successful as we think at eradicating H. pylori.
Although several possibilities exist for treatment failures one important concern is antibiotic resistance. Specifically, the decline in treatment efficacy has in part been due to increasing resistance of H. pylori to clarithromycin. This trend, now being observed in many industrialized countries, reflects at least in part the growing use of second generation macrolides, up 388% in the US from 1992 to 2000 (10).
To avoid the unnecessary use of antibiotics, the indication for H. pylori testing should be clear since the evidence is strongest for PUD, and gastric MALT-type lymphoma. In the setting of dyspepsia the actual beneï¬t of eradication is questionable, given a less than 10% symptom improvement rate at 3 to 12 months after treatment(11). In order to reduce the chance of an adverse outcome in individuals with an appropriate indication for eradication and to limit the pool of antibiotic resistant strains, the current consensus is that H. pylori eradication should be confirmed post treatment. Non-invasive testing using the urea breath test or the stool antigen test, are appropriate for confirmation. This can be done within 6 weeks of treatment with the patient off proton pump inhibitors for two weeks.
Many practitioners often debate the need to treat H. pylori only AFTER obtaining confirmation of colonization. The following simplified approach has been recommended:
Do not test for H. pylori unless the indication is clear and you plan to treat
Do not treat H. pylori unless you plan to confirm eradication
The importance of confirming H. pylori eradication is can be summarized as follows:
It reassures the patient about eradication of a class I carcinogen.
It provides proof that the risk of complications has been eliminated.
It allows for re-treatment if necessary.
It reduces the risk of propagating resistant pools with treatment failures.
The current primary therapies for H. pylori include a proton-pump inhibitor (PPI), clarithromycin, and amoxicillin, or metronidazole for 14 days. Another option is a PPI, bismuth, metronidazole, and tetracycline for 14 days. Sequential therapy consisting of a PPI and amoxicillin for 5 days followed by a PPI, clarithromycin, tinidazole for 5 days has been shown to be effective in European studies but has not been validated in the U.S.
What should be the approach to patients who remain colonized after an initial treatment regimen? One approach for second-line treatment is quadruple therapy with a proton-pump inhibitor, colloidal bismuth subcitrate, metronidazole, and tetracycline (12) if not used as first-line. Another approach is to avoid antibiotics used in the first-line regimen, in particular clarithromycin. Levofloxacin-based triple therapy for 10 days has shown promise as an effective salvage regimen (13). Routine pretreatment genotyping or susceptibility testing, although not currently recommended, may be useful for individuals whose second-line treatment has failed since it may help direct the selection of an appropriate rescue regimen (14).
References:
[1] Everhart JE. Recent developments in the epidemiology of Helicobacter pylori. Gastroenterol Clin North Am. 2000, 29(3):559-78
[2] Leung WK, Lin SR, Ching JY, To KF, Ng EK, Chan FK, Lau JY, Sung JJ. Factors predicting progression of gastric intestinal metaplasia: results of a randomised trial on Helicobacter pylori eradication.Gut. 2004 Sep;53(9):1244-9
[3] Paptheodoridis GV, Sougioultzis S, Archimandritis AJ. Effects of Helicobacter pylori and nonsteroidal anti-inflammatory drugs on peptic ulcer disease: A sys- tematic review. Clin Gastroenterol Hepatol 2006;4:130- 42
[4] Nomura AM, Pérez-Pérez GI, Lee J, Stemmermann G, Blaser MJ. Relation between Helicobacter pylori cagA status and risk of peptic ulcer disease.Am J Epidemiol. 2002 Jun 1;155(11):1054-9.
[5] Parsonnet J, Hansen S, Rodriguez L, Gelb AB, Warnke RA, Jellum E, Orentreich N, Vogelman JH, Friedman GD. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994 May 5;330(18):1267-71.
[6] Mera R, Fontham ETH, Bravo LE, et al. Long term follow-up of patients treated for Helicobacter pylori infection. Gut 2005;54:1536-40.
[7] Leodolter A, Kulig M, Brasch H, et al. A meta-analysis comparing eradication, healing and relapse rates in patients with Helicobacter pylori-associated gastric or duodenal ulcer. Aliment Pharmacol Ther 2001;15:1949-58.
[8] Montalban C, Norman F. Treatment of gastric mucosa-associated lymphoid tissue lymphoma: Helicobacter pylori eradication and beyond. Expert Rev Anticancer Ther. 2006;6:361-71.ulcer-like functional dyspepsia. Aliment Pharmacol Ther 2001;15:195-201.
[9] Laheij RJ, Rossum LG, Jansen JB, Straatman H, Verbeek AL. Evaluation of treatment regimens to cure Helicobacter pylori infection–a meta-analysis. Aliment Pharmacol Ther. 1999 Jul;13(7):857-6
[10] McCaig LF, Besser RE, Hughes JM. Antimicrobial drug prescription in ambulatory care settings, United States, 1992-2000. Emerg Infect Dis. 2003 Apr;9(4):432-7.
[11] Moayyedi P, Soo S, Deeks J, Delaney B, Harris A, Innes M, et al. Eradication of Helicobacter pylori for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2005:CD002096.
[12] Malfertheiner P, Me´graud F, O’Morain C, Hungin AP, Jones R, Axon A, et al. Current concepts in the management of Helicobacter pylori infection-the Maastricht 2-2000 Consensus Report. Aliment Pharmacol Ther. 2002;16:167- 80.
[13] Saad RJ, Schoenfeld P, Kim HM, Chey WD. Am J Gastroenterol. 2006 Mar;101(3):488-96.
[14] Francois F, Blaser MJ. Improving Helicobacter pylori eradication regimens.Ann Intern Med. 2006 Jan 17;144(2):1401.
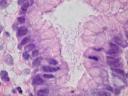
Image courtesy of Wikimedia Commons, Helicobacter pylori. Dozens of the curved bacteria fill the lumen of a gastric foveola. Hematoxylin & eosin stain, 1000X.
