Peer Reviewed
Last week the US team bowed out of the World Cup, but not without a valiant final effort. Particularly noteworthy was goalie Tim Howards’ performance, leading some fans to affectionately label him the new “Secretary of Defense” (1). In spite of the US team’s exit from the tournament, “the beautiful game”, as the great Brazilian player Pelé once called it, continues to both entertain and teach in unexpected ways. As proof of the latter, we begin this week’s Primecuts with an article published in The Lancet Infectious Diseases about the risk of Dengue fever in Brazil, home to this year’s tournament.
An early warning model framework for Dengue and the World Cup
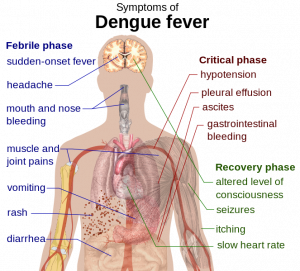
This century, Brazil has had more reported cases of dengue fever than any other country in the world. The World Cup is currently taking place in 12 different cities throughout the nation and is expected to attract over one million spectators. This, in turn, prompted concerns of a dengue fever epidemic and led a multi-national team to create a probabilistic forecast of dengue risk for the 12 game-hosting cities (2). The team gathered data for multiple contributing factors, including infection trends, social/environmental variables, and real-time seasonal climate forecasts, and used them to create a modeling framework that allowed dengue warnings to be made three months in advance of any given date.
The probability of dengue incidence was calculated under 3 predefined categories: low (100 cases per 100,000 inhabitants), medium (100-300 cases per 100,000) and high risk (>300 cases per 100,000). Based on this analysis, cities in southern Brazil and parts of the Amazon rainforest (eg, Sao Paulo, Brasília, etc) were deemed low risk, whereas cities in the Northeast (Recife, Fortaleza and Natal) were labeled high risk. Cities in other regions (Rio de Janeiro, Manaus, etc) were deemed medium risk. The study was intended to identify optimum trigger alert thresholds, and thus hopefully help local agencies focus on interventions to control mosquito populations in high risk cities. However, there are some limitations to the model’s use, including the basic fact that such efforts need to be in place before visitors arrive -raising the question of whether the article was published too late and whether such efforts will be sustainable.
The effects of statins on secondary progressive multiple sclerosis
Moving on to news in Neurology, The Lancet published the results of a phase 2 trial studying the effects of high dose simvastatin on brain atrophy in patients with secondary progressive multiple sclerosis (MS) (3). This double blind, randomized placebo-controlled trial took place in England from 2008 to 2011. One-hundred and forty patients with secondary progressive MS were randomized to receive 80mg Simvastatin or placebo. The primary endpoint was the rate of whole-brain atrophy per year as measured by volumetric MRI at baseline, 12 and 25 months. Secondary endpoints were number of new and enlarging T2 brain lesions, clinical outcomes, and changes in multiple serum markers of inflammation. The mean atrophy rate on imaging was 0.288% (SD 0.521) per year in the simvastatin group compared to 0.584% (SD 0.498) for placebo, representing a rate difference of -0.254% per year (95% CI, -0.422 to -0.087; p=0.003). Similar results were noted between baseline to 12 months, and baseline to 25 months. There was no difference noted between groups in terms of inflammatory markers, new/enlarging lesions, or rates of relapse.
The study, however, is not without its flaws: the definition of “secondary progressive multiple sclerosis” (a diagnostic requirement for inclusion) is implied but never clearly stated. Medication compliance was largely self-reported, and baseline characteristics of both groups were not always well matched. Overall, the study showed promising results on imaging but, by the author’s own admission, care should be exercised when interpreting brain imaging findings as these may not necessarily correlate with clinical outcomes.
Effects of erythropoietin and pRBC transfusion on neurological recovery after TBI
The Journal of the American Medical Association (JAMA) published a randomized trial analyzing the effects of IV erythropoietin (EPO) and hemoglobin (Hb) transfusion targets on neurological recovery after traumatic brain injury (TBI) (4). On admission, patients were first randomized to EPO vs placebo. Once ICP monitoring and ventilatory support were no longer required, they were then randomized to Hb transfusion goals of 7 or 10 g/dL (independently of EPO/placebo allocation). A neurological baseline was established using the Glasgow Coma Scale after initial resuscitation and outcomes were measured using the Glasgow Outcome Scale at 6 months. Results were dichotomized into “favorable” (good recovery or moderate disability) or “unfavorable” (severe disability, vegetative or dead).
Unfortunately, the study faced several limitations. Among other challenges, the EPO dosing regimen was modified midway through the study due to safety concerns raised by the FDA based on the results of a separate trial (5). This not only delayed the study, but also forced the authors to modify their design from a superiority trial to a futility trial that ultimately concluded that EPO (including both dosing regimens) was futile vs placebo. Favorable GOS scores at 6 months were 38.2% for placebo (95% CI, 28.1% to 49.1%), 48.5% for EPO1 (95% CI, 31.4% to 66%, p=0.13) and 29.8% for EPO2 (95% CI, 18.4% to 43.4%, p<0.001).] There was also no significant difference in favorable outcomes between the 7 and 10g/dL hemoglobin thresholds (42.5%, for a 7g/dL threshold and 33% for the 10g/dL threshold [95% CI, -0.06 to 0.25, p=0.28]). Furthermore, a higher incidence of thromboembolic events was seen with the transfusion threshold of 10g/dL (21.8% vs 8.1%, OR 0.32, 95% CI, 0.12 to 0.79, p=0.009). So regardless of the many difficulties in interpretation and analysis of this study, the published data cannot support the use of EPO or the use of a higher 10g/dL Hb transfusion threshold in patients following TBI -if anything, it raised concerns over the higher Hb threshold.
The peer review process and its impact on the reporting of results of randomized trials
The British Medical Journal published a retrospective study investigating the effectiveness of open peer review as a mechanism to improve the reporting of randomized trials (6). Utilizing the Consolidated Standards of Reporting Trials (CONSORT) checklist, the team examined whether specific methodological aspects of the trials were reported. They then evaluated changes in reporting of these items between the original and final versions of a total of 93 studies. The types of changes requested and the extent to which authors adhere to the reviewer’s requests were also reviewed.
Results showed that when reviewers requested changes to reporting of methods, most writers made these changes (e.g., 93% of comments regarding trial design and 93% regarding primary outcome results lead to changes, etc). However, 55% of articles failed to adequately report results for primary or secondary outcomes as well as other significant aspects such as blinding (50%) in either the original or final version –implying that both the authors and the reviewers missed these. Most of the changes requested by reviewers had a “positive” impact, but some had a “negative” impact; including reviewers requesting changes to the primary outcome, adding new secondary outcomes, or over-inflating conclusions. It should be noted that “positive” and “negative” are never clearly defined in the study, other than the implication that “positive” means that a clarification on the reporting of methodology was made to the text, and “negative” includes reviewers making inappropriate requests such as the aforementioned examples.
The team concluded that peer reviewers often fail to detect important deficiencies in the reporting of methodology and results of trials and that reviewers on occasion even made inappropriate requests. This raises questions regarding the inconsistencies and inadequacies of the peer review process. The study is limited in that its analysis focused strictly on the reporting of methodological aspects (as opposed to the reporting of clinical data). Lastly, the sample was limited to articles from a single medical journal that utilizes open peer review, and it is unknown whether the open process itself may hinder the reviewer.
ALSO IN THE JOURNALS THIS WEEK
— The sunscreen counseling practices of US physicians are analyzed in a study published in JAMA (7). Although sunscreen is known to be a key aspect of skin-cancer prevention, the study questions if outpatient physicians are addressing this issue with patients as often as is needed.
— A review of human schistosomiasis is presented in The Lancet (8). The article includes information on epidemiology, pathogenesis, diagnosis and treatment, along with a review of control and elimination strategies.
— The International Journal of Antimicrobial Agents published a review article on the pathogenicity and treatment of Bartonella Infections (9). There is currently no available single treatment that is effective against all Bartonella-associated diseases. With Bartonella human infectious caused predominantly by B henselae, B quintana and B bacilliformis, clinical manifestations range from self-limited to life-threatening disease.
— The New England Journal of Medicine published an online progress report on changes to health care under the Affordable Care Act (10), including an assessment of the events to date, the enrollment through individual marketplaces, expansion of Medicaid eligibility, and new insurance-market rules.
Dr. Miguel A. Saldivar is a second year resident at NYU Langone Medical Center
Peer reviewed by Jessica Taff, MD, Associate Editor, Clinical Correlations
Image courtesy of Wikimedia Commons
References
1. “Tim Howard. Ten tweets about a new US hero”. http://www.bbc.com/news/world-us-canada-28122254. July 1, 2014.
2. Rachel Lowe, PhD, Christovam Barcellos, PhD, Caio A S Coelho, PhD, et al. Dengue outlook for the World Cup in Brazil: an early warning model framework driven by real-time seasonal climate forecasts. The Lancet Infectious Diseases. Volume 14, Issue 7, July 2014, Pages 619–626. DOI: 10.1016/S1473-3099(14)70781-9. http://www.sciencedirect.com.ezproxy.med.nyu.edu/science/article/pii/S1473309914707819
3. Jeremy Chataway, PhD, Nadine Schuerer, PhD, Ali Alsanousi, PhD, et al. Effect of high-dose simvastatin on brain atrophy and disability in secondary progressive multiple sclerosis (MS-STAT): a randomised, placebo-controlled, phase 2 trial. The Lancet. Volume 383, Issue 9936, 28 June–4 July 2014, Pages 2213–2221. DOI: 10.1016/S0140-6736(13)62242-4. http://www.sciencedirect.com.ezproxy.med.nyu.edu/science/article/pii/S0140673613622424
4. Effect of Erythropoietin and Transfusion Threshold on Neurological Recovery After Traumatic Brain Injury. A Randomized Clinical Trial. Claudia S. Robertson, MD; H. Julia Hannay, PhD; José-Miguel Yamal, PhD, et al. JAMA. 2014;312(1):36-47. doi:10.1001/jama.2014.6490. http://jama.jamanetwork.com.ezproxy.med.nyu.edu/article.aspx?articleid=1884575
5. Ehrenreich H,Weissenborn K, Prange H, et al; EPO Stroke Trial Group. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke. 2009;40(12):e647-e656. PMID: 19834012. http://www.ncbi.nlm.nih.gov/pubmed/19834012
6. Sally Hopewell, Gary S Collins, Isabelle Boutron, et al. Impact of peer review on reports of randomised trials published in open peer review journals: retrospective before and after study. BMJ 2014; 349 doi: http://dx.doi.org/10.1136/bmj.g4145 (Published 01 July 2014) Cite this as: BMJ 2014;349:g4145. http://www.bmj.com/content/349/bmj.g4145
7. Daniel G. Federman, MD; Robert S. Kirsner, MD, PhD; John Concato, MD. Sunscreen Counseling by US Physicians. JAMA. 2014;312(1):87-88. DOI: 10.1001/jama.2014.4320. http://jama.jamanetwork.com.ezproxy.med.nyu.edu/article.aspx?articleid=1884554
8. Dr Daniel G Colley, PhD, Amaya L Bustinduy, MD, W Evan Secor, PhD, et al. Human schistosomiasis. The Lancet Volume 383, Issue 9936, 28 June–4 July 2014, Pages 2253–2264. DOI: 10.1016/S0140-6736(13)61949-2. http://www.sciencedirect.com.ezproxy.med.nyu.edu/science/article/pii/S0140673613619492
9. Emmanouil Angelakis, Didier Raoult. Pathogenicity and treatment of Bartonella infections. International Journal of Antimicrobial Agents, Volume 44, Issue 1, July 2014, Pages 16–25. DOI: 10.1016/j.ijantimicag.2014.04.006 http://www.sciencedirect.com.ezproxy.med.nyu.edu/science/article/pii/S0924857914001186
10. David Blumenthal, M.D., M.P.P., Sara R. Collins, Ph.D. Health Care Coverage under the Affordable Care Act – A Progress Report. July 2, 2014DOI: 10.1056/NEJMhpr1405667. http://www.nejm.org.ezproxy.med.nyu.edu/doi/full/10.1056/NEJMhpr1405667?query=featured_home