 Commentary By: Minisha Sood PGY-3
Commentary By: Minisha Sood PGY-3
The FDA has received reports of 90 patients with moderate to end-stage kidney disease who have undergone MRI or MRA with a gadolinium-based contrast agent and subsequently developed a new disease known as Nephrogenic Systemic Fibrosis (NSF).
Scientists first identified NSF, also known as Nephrogenic Fibrosing Dermopathy (NFD), in 1997 and its cause has not yet been identified. There have been approximately 200 reports of NSF/NFD only in people with kidney disease. Neither the duration of kidney disease nor its underlying cause is related to the development of NSF.
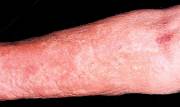
Patients with NSF report swelling and tightening of the skin, which usually affects the extremities and leads to an inhibition of flexion and extension. Muscle weakness is a common symptom as well. Approximately 5% of patients experience a rapidly progressive course, which may result in death due to widespread fibrosis. The pathogenesis of NSF is thought to be linked to the circulating fibrocyte (CF), a recently characterized cell that is distinct from a fibroblast. The CF leaves the circulation, the mechanism of which is still being investigated, and differentiates in the dermis where it resembles normal fibroblasts and leads to a systemic disorder.
Many approaches to treatment are currently under investigation including oral steroids, physical therapy, plasmapheresis, high-dose IVIG therapy, and renal transplantation.
Here is some important information for health care providers:
- When a patient with moderate to end-stage kidney disease requires an imaging study, a modality other than MRI or MRA with a gadolinium-based contrast agent should be chosen whenever feasible.
- If MRI or MRA with a gadolinium-based contrast agent is necessary, it may be prudent to arrange prompt dialysis for patients with advanced kidney dysfunction.
- The FDA requests that health care providers and patients report adverse event information to the FDA online at http://www.fda.gov/medwatch/report.htm, by phone (1-800-FDA-1088) or by fax (1-800-FDA-0178).