 Commentary by Judith Brenner MD, Associate Program Director, NYU Internal Medicine Residency Program
Commentary by Judith Brenner MD, Associate Program Director, NYU Internal Medicine Residency Program
There were many medical stories in the news last week that seemed relevant and worthy of a spot in this week’s ShortCuts. First, the CDC released a statement regarding the flu in its February 9th issue of MMWR. The CDC conducts surveillance in several ways and this year appears to have more states reporting flu activity and more specimens positive for influenza than in the last 3 years. The question has arisen about the degree of match between the circulating viruses and the vaccine strains, but the jury remains out. For now, the CDC continues to recommend vaccination for prevention. In addition, it continues to recommend oseltamivir and zanamivir for treatment and prophylaxis. Of note, there has been a slight increase in the resistance of influenze to oseltamivir (now at 5.9% of specimens tested).
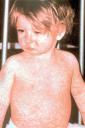 Staying in the world of infectious disease, but perhaps even closer to home, the NYC Health Department released a warning to doctors to remain alert to the possibility of measles after 2 cases were identified in Brooklyn. As a reminder, think of measles in people who visit from overseas or those who are unvaccinated and in potential contact (mucosal transmission) with someone from overseas. The illness is marked by fever, cough, conjunctivitis, and rhinorhea. Most notable is the rash, which begins on the face and moves down and includes the palms and soles.
Staying in the world of infectious disease, but perhaps even closer to home, the NYC Health Department released a warning to doctors to remain alert to the possibility of measles after 2 cases were identified in Brooklyn. As a reminder, think of measles in people who visit from overseas or those who are unvaccinated and in potential contact (mucosal transmission) with someone from overseas. The illness is marked by fever, cough, conjunctivitis, and rhinorhea. Most notable is the rash, which begins on the face and moves down and includes the palms and soles.
So, we’ve covered: 1. NYC and measles 2. The US and influenza What’s left? How about the world and MDR-TB? In the latest issue of the Lancet, an editorial was written regarding the WHO published survey on the global spread of MDR-TB. It is estimated that there are nearly 1/2 million cases annually of MDR-TB with about 20% of these cases resulting in death. This includes many cases of XDR-TB–extensively drug resistant. The survey was the largest of its kind and included 30 countries that had previously not reported drug resistance. Of particular concern is the combination of MDR-TB among those HIV infected. Of note, the survey does not include extensive data from Africa.
The last newsworthy item regards the recall of heparin, including, most recently, the heparin used in “Hep-Locks.” Initially, increased reports of allergic reaction were noted as well as four deaths. In response, Baxter International stopped production. (Baxter is one of the main manufacturers of heparin. A US company has agreed to increase its production to avert a heparin shortage.) The problem is currently traced to a Chinese factory, where procedural problems have been noted in how the pig intestine, the main ingredient in heparin, is harvested. Stay tuned for more details.
Lastly, in the most recent issue of the Annals of Internal Medicine, Rozendaal et al reported on the “Effect of Glucosamine Sulfate on Hip Osteoarthritis.” In this study, 222 patients with relatively early stage hip OA were treated with 2 years of oral glucosamine sulfate or placebo. The primary outcome was the WOMAC pain and function scores (very common in studies of this sort) and change in joint space narrowing was a secondary endpoint. There were no differences found in any of these parameters after 2 years of study. So, when our patients come in asking us about glucosamine, what should we say? First, keep in mind that the vast majority of studies on glucosamine have been done on knee, rather than hip OA. The accompanying editorial reminds us that the two diseases are different, with knee OA likely being more “inflammatory” in comparison. Secondly, because glucosamine is considered a dietary supplement rather than a drug by the FDA, it is not subject to the same requirements for standardization and purity of its contents. Nevertheless, in the largest systematic review–a Cochrane–a benefit was found. The only formation that showed any potential benefit was the “Rottapharm” preparation. (Notably, this was NOT the preparation used in the study reported here). Three studies published after the Cochrane review showed conflicting results. Thus, as a clinician, I’m left unsure. Is there a downside? In this study, 52.3% of patients reported adverse events, though only 7% dropped out. Although no significant difference was found between placebo and glucosamine, the study was underpowered to demonstrate a true difference. So, in summary, you have at least two preparations, a heterogeneous disease and multiple conflicting trials….
