Faculty Peer Reviewed
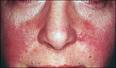
Ms. W is a 35 yo woman with a history of systemic lupus erythematosus diagnosed 10 years ago. Her only medications are hydroxychloroquine and prednisone for occasional disease flares. She is otherwise healthy. She has no known personal or family history of cardiac disease or stroke, but does smoke ½ pack of cigarettes per day. Currently, she denies any chest pain, shortness of breath, urinary symptoms, lower extremity edema, or menstrual irregularities, but does report occasional mild joint pain and photosensitivity. Physical exam is notable for normotension, one 2mm shallow ulcer on the hard palate, faint erythema of the nasolabial folds bilaterally, and mild truncal obesity (BMI-28).
Systemic lupus erythematosus is an autoimmune disorder affecting roughly 250,000 Americans.[1] The hallmark of the disease is antibodies directed against cellular nuclear components. The most common clinical features that physicians encounter include dermatologic changes, arthritis, and nephritis. However, a relationship between SLE and cardiovascular disease has been repeatedly noted. Case reports of SLE patients developing premature coronary artery disease date back to the 1960s. Today, with these patients living longer due to more effective drug therapies, ischemic heart disease has become a leading cause of late mortality.
The first observations regarding late mortality due to myocardial infarction came out in 1976.[2] Several other studies have confirmed this association, most notably a landmark cohort study done by Susan Manzi and her colleagues, published in 1997.[3] In their paper they compared cardiovascular event rates between SLE patients at the University of Pittsburgh lupus clinic from 1980 to 1993 with normal patients in the Framingham Offspring Study. They found that women aged 35-44 were over 50 times more likely to suffer a myocardial infarction than aged-matched controls (OR-52.43). The increased risk declined for older patients, but was still significant. For women aged 45-54, the odds ratio was 2.47; and for women aged 55-64, the odds ratio was 4.21. Furthermore, other studies have shown that SLE patients have higher rates of sub-clinical atherosclerosis. Numerous case-control studies-both autopsy studies and myocardial perfusion studies-have consistently shown a 30-40% prevalence of sub-clinical CAD in these patients.[4]
The pathophysiology underlying these statistics, although not completely understood, is thought to be related to both traditional and non-traditional risk factors for CAD. The traditional risk factors (demographics, family history, smoking status, hypertension, diabetes, dyslipidemia) were elucidated in the Framingham study. The non-traditional risk factors include chronic inflammation and the presence of autoantibodies. Prolonged vascular inflammation, corticosteroid use, renal disease, hypertension, and antiphospholipid antibodies all play a role in plaque formation. Interestingly, in the Manzi study the women that were more likely to develop CAD had the following characteristics: older age at lupus diagnosis (which could point to a longer period of untreated sub-clinical lupus and vascular damage); longer disease duration; higher total steroid use over time; hypercholesterolemia; and post-menopausal state.[3]
One factor that has repeatedly been shown to affect the prevalence of CAD is disease control. One study published in 2003 demonstrated that better control of disease activity correlated with a lower incidence of carotid atherosclerosis.[5] In this case-control study, patients taking hydroxychloroquine or cyclophosphamide had significantly less carotid atherosclerosis than those patients not on these medications. Furthermore, those patients with mean higher daily prednisone use were also less likely to have plaque than those with lower mean daily steroid use. Both of these observations suggest that more aggressive control of disease activity could result in lower plaque burden.
Patients with SLE also develop dyslipidemia. The typical “lupus pattern” is high VLDL, high triglycerides, and low HDL, even in dormant disease. This profile would obviously potentiate any patient’s risk of CAD. However, even after taking into account these traditional risk factors, SLE patients still have higher rates of atherosclerotic disease, providing evidence that the Framingham risk calculator may be a poor predictor of 10-year cardiovascular risk in this population.
To account for this discrepancy and to look for better markers of CAD, much research has been done on other laboratory tests, the so-called “novel risk factors”. One such marker is homocysteine. Homocysteine promotes platelet aggregation and intimal thickening, both of which directly contribute to plaque formation. In the Toronto Risk Factor Study of 2003, SLE patients were more likely to have elevated homocysteine levels than matched controls, and hyperhomocysteinemia was found to be an independent risk factor for thromboembolic stroke and arterial thrombosis.[6] Of note, patients and controls had equivalent Framingham risk scores for 10 year coronary event rates. Other studies have not confirmed these findings, however.[5] Folate supplements can lower homocysteine levels, but studies showing a mortality benefit from this treatment are currently lacking.
Another area of research is C-reactive protein. There are conflicting studies about the correlation between CRP and atherosclerosis. While both the Physician’s Health Study and the Women’s Health Study demonstrated that elevated CRP levels were associated with increased risk of MI and ischemic stroke, other studies have failed to show this relationship. Although lupus patients tend to have higher CRP levels than controls, this has not been shown to correlate strongly with evidence of atherosclerosis.[5] The role of CRP as a marker of atherosclerosis is still an area of future inquiry.
Other biochemical markers being studied include intercellular adhesion molecule-1 (ICAM-1) and vascular-cell adhesion molecule-1 (VCAM-1), both of which are involved in the inflammatory stage of plaque formation.[5,7] Also, there remains the ongoing question of whether the pro-atherogenic properties of antiphospholipid antibodies are potent enough to warrant consideration as an independent risk factor for atherosclerosis.
Due to the high prevalence of CAD in SLE patients, SLE itself should be viewed as a coronary risk factor in the same way that diabetes is. Consequently, these patients should be adequately screened for modifiable risk factors and appropriate interventions should be made. Of primary importance are control of hypertension, diabetes, and lipids; smoking cessation; and weight loss for obese patients.
Patients should have an annual fasting blood glucose and a urinalysis at every clinic visit to assess for proteinuria, glucosuria, and hematuria. Those patients with evidence of impaired glucose tolerance should undergo dietary changes to prevent frank diabetes from developing.
Blood pressure should be followed at every visit with a goal BP of <130/80. For pre-hypertensive patients, the physician should first try therapeutic lifestyle changes (exercise, diet modification) and assess renal function. If BP is consistently above 140/90 despite TLC, anti-hypertensive medications should be started. Patients may require two drugs to reach this goal. ACE-inhibitors and calcium channel blockers (which may help with Raynaud’s phenomenon) are first line agents. Both enalapril and amlodipine have been shown to slow the progression of carotid plaques in patients with NIDDM, but their effect in SLE has not been studied.[7] Extrapolation would suggest a benefit in SLE patients as well. Beta blockers should be used with caution as they may precipitate Raynaud’s.
Similar to diabetics, the cholesterol recommendations for lupus patients are more stringent than those for the average patient.[8] SLE patients should have an annual fasting lipid profile, with a goal LDL <100mg/dL. For LDL 100-129, therapeutic lifestyle changes should be tried, with a repeat lipid profile in 3-6 months. Statin therapy is indicated for LDL >130, even in those without traditional coronary risk factors. Statins have been shown to directly improve endothelial function, even in patients with normal lipid levels. Furthermore, antimalarial drugs, like hydroxychloroquine, can also improve a patient’s lipid profile by lowering both LDL and VLDL.
Also, these patients should be counseled on smoking cessation and weight reduction if their BMI >25. Low-dose aspirin should be considered for those patients with traditional coronary risk factors and those that are antiphospholipid antibody positive.
Although we have known of the very real correlation between premature coronary disease and SLE for several decades, the exact mechanisms behind this association are not fully understood. To prevent long-term cardiovascular consequences, these patients should be treated aggressively, both to control their primary lupus activity, and to minimize modifiable risk factors. As more research into this field continues, we will gain a better insight into how chronic inflammation contributes to coronary disease and hopefully, be better equipped to decrease the rate of late morbidity and mortality associated with ischemic heart disease.
Regarding the case of Ms. W, she should be advised to stop smoking and attempt weight loss with diet modification and exercise. Blood pressure should be checked and lowered to a goal of <130/80. A fasting lipid panel and blood glucose should be ordered with interventions made to lower LDL to <100 and glucose <100. Renal function should be assessed with serum creatinine, urinalysis, and 24-hr urine protein, and she may benefit from an ACE-i. She should continue hydroxychloroquine, and should receive yearly fundoscopic exams. Serologies should also be checked: antiphospholipid panel, anti-ds-DNA, and complement levels. If she develops other risk factors, aspirin should be started.
Dr. Bradley is a 2nd year internal medicine resident at NYU Medical Center.
Peer reviewed by Peter Izmirly MD, NYU Division of Rheumatology
References:
1. Rahman, Anisur and David A. Isenberg. “Systemic Lupus Erythematosus.” N Engl J Med 2008;358:929-39.
2. Urowitz, MB et al. “The Bimodal Mortality Patten of Systemic Lupus Erythematosus.” Am J Med 1976;60:221-225.
3. Manzi, Susan et al. “Age-Specific Incidence Rates of Myocardial Infarction and Angina in Women with Systemic Lupus Erythematosus: Comparison with the Framingham Study.” Am J Epidemiol 1997;145:408-415.
4. Korkmaz, C, DU Cansu, and T Kasifoglu. “Myocardial Infarction in Young Patients (≤35 years of age) with Systemic Lupus Erythematosus: A Case Report and Clinical Analysis of the Literature.” Lupus 2007;16:289-297.
5. Roman, Mary J et al. “Prevalence and Correlates of Accelerated Atherosclerosis in Systemic Lupus Erythematosus.” N Engl J Med 2003;349:2399-2406.
6. Bruce, IN et al. “Risk Factors for Coronary Heart Disease in Women with Systemic Lupus Erythematosus: the Toronto Risk Factor Study.” Arthritis Rheum 2003; 48(11):3159-3167.
7. Nikpour, Mandana, Murray B Urowitz, and Dafna D. Gladman. “Premature Atherosclerosis in Systemic Lupus Erythematosus.” Rheum Dis Clin N Am 2005;31:329-354.
8. Wajed, J, et al. “Prevention of Cardiovascular Disease in Systemic Lupus Erythematosus – Proposed Guidelines for Risk Factor Management.” Rheum 2004;43:7-12.