 By Michael C. Brabeck, MD, Adam Davis, MD, and Shaun Rodgers, MD
By Michael C. Brabeck, MD, Adam Davis, MD, and Shaun Rodgers, MD
Faculty Peer Reviewed
Case:
A 56-year-old male was admitted to Bellevue Hospital Center with chronic back pain and a three month history of subjective fevers, decreased appetite, and a thirty pound weight loss. His past medical history was remarkable only for chronic alcohol abuse. He denied other substance abuse. There were no previous health care facility exposures, and the patient had no history of military service. The physical examination upon admission was notable for a cachectic appearing middle-aged Caucasian male. He was afebrile, and other vital signs were unremarkable except for mild hypertension. There was no external sign of trauma. Gait and neurological examination of his lower extremities were normal, as was his rectal sphincter tone. The remainder of physical examination was within normal limits.
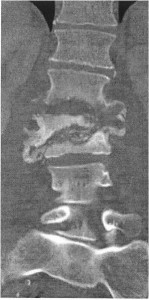
A normochromic, normocytic anemia was present. Erythrocyte sedimentation rate was 40 mm/h. Urine analysis was unremarkable. Plain films of the lumbar spine showed destruction of L1-3 vertebral bodies. CT imaging of the lumbar spine on HD 1 demonstrated multilevel discitis; vertebral osteomyelitis involving L1-3, subsequently confirmed by MR (Figure); and an adjacent discrete left psoas fluid collection at L-3, assumed to be a psoas abscess. Vancomycin and piperacillin/tazobactam were begun. Culture of percutaneous bone biopsy of the L-3 vertebral body done at that time yielded pansensitive E coli. At surgery for debridement and spinal fixation six days after admission, a left psoas abscess was confirmed. The L2-3 vertebral bodies and L2-3 interspace were involved with a destructive process producing exuberant pus. Culture of vertebral bone obtained at surgery yielded Acinetobacter baumannii, resistant to all antibiotics except for ampicillin/sulbactam. (Sensitivity testing to polymixin and tigecycline was not performed.) Empiric antibiotic therapy was discontinued, and ampicillin/sulbactam was begun.
Discussion
Vertebral osteomyelitis, psoas abscess, and spinal epidural abscess, are inter-related infections. Each share the same risk factors, and each of them may result from either a local, contiguous source, or from hematogenous dissemination.[1] While spinal epidural abscess and psoas abscess are often thought to result from vertebral osteomyelitis, each of these infections may result in any of the others.
Vertebral osteomyelitis is primarily a disease of adults. Its prevalence increases with age. Most commonly occurring by hematogenous spread, it is believed that the increasing incidence of vertebral osteomyelitis is due to the more frequent use of indwelling vascular catheters and the injection of recreational drugs. Less commonly, vertebral osteomyelitis can be caused by direct inoculation during spinal surgery or trauma, or as a result of spread from contiguous infections in soft tissues such as bowel or urinary tract.
Psoas abscess is also generally classified as primary or secondary.[2] Primary psoas abscess occurs in the absence of local causes, and is thought to have a hematogenous origin. In the past few decades, there has been an increase in the number of primary psoas abscesses relative to the secondary forms. Risk factors for primary psoas abscess are intravenous drug use, indwelling vascular catheters, and immunosupression (HIV, autoimmune disease, cirrhosis, alcoholism, cytotoxic chemotherapy or corticosteroids, etc).[3] The most commonly cultured organism in the primary form of psoas abscess is Staphylococcus aureus (63-80%).[4,5] In areas where tuberculosis is endemic, Mycobacterium tuberculosis is a frequent cause of primary psoas abscess.[6]
Secondary psoas abscess is associated with contiguous spread from gastrointestinal causes (Crohn’s disease, appendicitis, ulcerative colitis, diverticulitis); genitourinary causes (such as urinary tract infection or instrumentation); vertebral osteomyelitis; trauma; and occasionally, childbirth. The most frequently encountered organisms in secondary psoas abscess are gram negative enteric organisms.
The anatomy of the psoas muscle makes it susceptible to infection by both hematogenous seeding and direct extension. The psoas muscle originates from the lateral borders of T12 through L5, coursing through the pelvic retroperitoneum and over the pelvic brim to attach on the lesser trochanter. Its location places it in close proximity to the kidneys, ureters, pancreas, appendix, small intestine, and colon. The psoas is enveloped in a fibrous sheath, and psoas abscess resulting from GI or GU tract infections typically occur outside and adjacent to this sheath. Hematogenously disseminated infection, on the other hand, occurs within the sheath
Originally described in the 1930s, Acinetobacter is a gram negative coccobacillus. The species A. baumanii is the most frequent pathogen in human infections. Its natural habitat is soil and water, and it thrives in warmer temperatures. In humans, Acinetobacter can colonize the skin, respiratory tract, GI tract, and wounds.[7] Over the past three decades, Acinetobacter has emerged as an important hospital pathogen.
Acinetobacter flourishes in the warm and humid environment of intensive care units. In this setting it has become an important nosocomial infection, causing mainly endovascular infections and ventilator–associated pneumonia in critically ill patients, particularly in those with a prolonged ICU stay.7 Acinetobacter infections often occur as point source outbreaks, traced to contaminated respiratory therapy or ventilator equipment, and sometimes to contaminated hands of health care workers who have had contact with patients colonized or infected with the organism.[8] Risk factors for developing Acinetobacter infections include central vascular lines, tracheostomy, mechanical ventilation, enteral feedings, debilitated state, and recent surgery. Another documented risk factor is recent use of fluoroquinolones, third generation cephalosporins, and carbapenem antibiotics.9
Acinetobacter is only occasionally a cause of infection outside of a health care setting, especially in temperate climates. As a community acquired infection, it occurs more often in tropical climates, particularly during the rainy season, which provide the moisture and warmth necessary for a natural reservoir for this organism. The most common community acquired Acinetobacter infection is pneumonia, often with positive blood cultures. [9] Recently, however, both bone and soft tissue Acinetobacter infections have been reported in US military personnel injured in Iraq.[10m11]
Acinetobacter is a rare cause of vertebral ostemyelitis, spinal epidural abscess, or psoas abscess. The first report of Acinetobacter vertebral osteomyelitis occurred in the Spanish literature in 1985, and described its occurrence in a twenty-eight-year-old female heroin user.[12] In 2005, a case report from the UK described a patient who developed Acinetobacter infection and edema of the epidural fat, presumably as a complication of a previously placed epidural catheter.[13] Finally, two reports, respectively, describe the occurrence of Acinetobacter psoas abscess[6] and vertebral osteomyelitis[14] in patients without apparent predisposing factors for infection with this organism.
Acinetobacter is typically resistant to many antibiotics. It is often reported as being susceptible to ampicillin/sulbactam, but it is susceptible only to the sulbactam portion of the complex. Current recommendations suggest caution with the use of ampicillin/sulbactam alone, however, given the frequent development of resistance to this antibiotic. Although carbapenems have been used successfully, there is concern over the world-wide emergence of carbapenem-resistant isolates of A. buamanii, which drastically limits the range of potential therapeutic agents for this infection[15]. The polymixins (resurrected after having been virtually abandoned in the 1960s because of concerns over nephro- and neurotoxicity), and tigecycline, are sometimes effective options for multidrug-resistant Acinetobacter.
In conclusion, Acinetobacter infection is typically a nosocomial occurrence, causing mainly endovascular and respiratory infections. More recently, it has been reported in soft tissue infections and osteomyelitis in victims of penetrating trauma. It is an occasional cause of community acquired pneumonia. Only four reports in the literature describe A. baumanii as a cause of spinal and paraspinal infections. A predisposing factor to vertebral osteomyelitis and psoas abscess in this patient may have been chronic alcohol abuse. Moreover, because the specimen yielding Acinetobacter was obtained in the OR six days after the first biopsy was performed in the interventional radiology suite, it is possible that the organism was nosocomial rather than community acquired. Nonetheless, physicians should be aware that Acinetobacter can occasionally cause spinal and related infections, with or without predisposing factors, and that for Acinetobacter infections caused by multidrug-resistant isolates, antibiotic choices may be quite limited.
Legend:
Contrast enhanced coronal CT view showing osseous destruction of the L1 through L3 vertebrae, characterized by bony loss, cortical erosion, fragmentation and severe wedging deformity of the L2 vertebrae with near complete collapse of the L3 vertebra (arrows). The normal intervertebral disc space architecture is destroyed and the disc spaces are widened suggesting fluid collection and or abscess.
Dr. Brabeck, Department of Medicine, New York University School of Medicine, Dr. Davis, Department of Radiology, New York University School of Medicine, and Dr. Rodgers, Department of Neurosurgery, New York University School of Medicine
Peer reviewed by Melanie Maslow, Section Editor, Infectious Disease, Clinical Correlations
References:
1. Van den Berge M. Marie S, Kuipers T, et al. Psoas abscess: report of a series and review of the literature. Neth J Med 2005;63(10):413-16 http://www.ncbi.nlm.nih.gov/pubmed/16301764
2. Santanella R, Fishman E, Lipsett P. Primary vs Secondary Iliopsoas Abscess. Arch Surg 1999;130:1309-13
3. Siddiq F, Chowfin A, Tight R, et al. Medical vs Surgical Management of Spinal Epidural Abscess. Arch Intern Med 2004;164:2409-12
4. Darouiche R. Spinal Epidural Abscess. NEJM 2006;355:2012-20
5. Taiwo B. Psoas Abscess: A Primer for the Internist. South Med J 2001;90(1):1-5
6. Tahsin Turunç, Tuba Turunç, et al. Retrospective Evaluation of 15 Cases with Psoas Abscesses. Mikrobiyol Bul 2009;43(1):121-5
7. Muñoz-Price L, Weinstein RA. Acinetobacter Infection. NEJM 2008;358:1271-9
8 . Villegas MV, Hartstein AI. Acinetobacter Outbreaks, 1997-2000. Infect Control Hosp EPidemiol 2003;24:284-95
9. Anstey NM, Currie BJ, Withnall KM. Community Acquired Acinetobacter in the Northern Territory of Australia. Clin Infect Dis 1992;14:83-91
10. Davis KA, Moran KA, McAllister K. Multidrug-Resistant Acinetobacter Extremity Infections in Soldiers. Emerg Infect Dis 2005;11(8):1218-24
11. Shafer JJ, Mangino JE. Multidrug-Resistant Acinetobacter baumannii Osteomyelitis from Iraq. Emerg Infect Dis 2008;14(3):512-4
12. Miralles R, Tapies A, et al. Espondilodiscitis séptica por Acinetobacter en una paciente heroinómana. Rev Clin Esp 1985;177(5):228-30
13. Athmaja TR, Sanders GM. An Unusual Presentation of Epidural Acinetobacter Infection. Reg Anesth Pain Med 2005;30(6):577-9 http://www.ncbi.nlm.nih.gov/pubmed/16326345
14. Perez-Lopez C, Villarejo-Ortega FJ, Carceller-Benito F, Goldman L. Spinal epidural abscess caused by Acinetobacter baumannii mimicking as a herniated lumbar disc. Rev Neurol 2005;40(2):98-101 http://www.ncbi.nlm.nih.gov/pubmed/15712164
15. Poirel L, Nordmann P. Carbapenem resistance in Acinteobacter baumanii: mechanisms and epidemiology. Clinical Microbiology and Infection 2006;12(9): 826-36 http://www.ncbi.nlm.nih.gov/pubmed/16882287