Podcast: Play in new window | Download
Subscribe: RSS
By Amy Shen Tang MD, Marty Fried MD and Shreya P. Trivedi MD; Illustration by Mike Natter MD. Quiz yourself on the following 5 Pearls on Chronic Hepatitis B (HBV)
Time Stamps
- What are the most common ways HBV is transmitted and how can we use this to prioritize screening? (3:37)
- Who is at risk for HBV reactivation?
- What tests do you order when screening for HBV? (10:48)
- In which populations is HBV vaccination recommended?
- What are the four possible meanings of an isolated positive total anti-HBc? (13:55)
- In an asymptomatic adult, is it necessary to send an anti-HBc IgM to distinguish acute from chronic HBV infection? (15:01)
- What are the implications of seroconversion of HBeAg from positive to negative? (16:19)
Subscribe to CORE IM on any podcast app! Follow us on Facebook @Core IM || Twitter @COREIMpodcast || Instagram @core.im.podcast. Please give any feedback at COREIMpodcast@gmail.com.
Show Notes
Pearl 1:
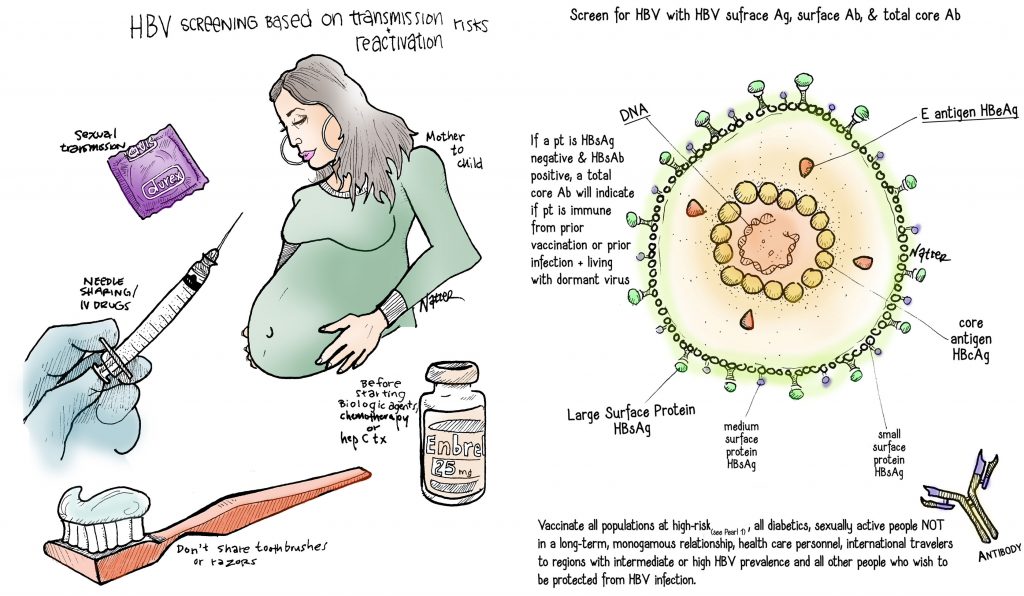
- HBV screening is based on transmission and reactivation risks.
- You can think about three high risk screening groups based on modes of HBV transmission:
- Mother to child: All people born in regions with > 2% HBV prevalence. This includes the entire continents of Asia and Africa, parts of South America, the Middle East, and Eastern Europe. Plus all pregnant women.
- Sexual transmission: Household contacts & sexual partners of people with HBV, men who have sex with men
- Needle-sharing: Current and former IV drug users, hemodialysis patients
- Before starting a biologic agent, chemotherapy or hepatitis C treatment, screen for HBV; these agents may predispose to HBV reactivation.
- HBV reactivation can cause liver failure and even death.
- Screen for HBV when starting treatment for HIV and HIV preexposure prophylaxis (PREP) as there are medications (such as tenofovir) used for both HIV and HBV.
- Fun Fact: HBV can live outside of the body in dried blood for at least 7 days!
- Counsel chronic HBV infected patients about NOT sharing toothbrushes or razors. All blood spills should be cleaned with bleach.
Pearl 2:
- Screen for HBV with HBV surface antigen (HBsAg), surface antibody (anti-HBs) and total core antibody (anti-HBc)
- If a patient is HBsAg negative and anti-HBs positive, a total core antibody will indicate if the patient is immune from prior vaccination or prior infection and living with the virus dormant in their liver, making them at a risk for reactivation
- Vaccinate all populations at high-risk (see Pearl 1), all diabetics, sexually active people NOT in a long-term, monogamous relationship, health care personnel, international travelers to regions with intermediate or high HBV prevalence and all other people who wish to be protected from HBV infection.
Pearl 3:
- Four meanings of a positive total anti-HBc with a negative anti-HBs and HBsAg:
- The majority of patients with an isolated total anti-HBc have been infected in the past and just have undetectable levels of anti-HBs
- You may consider measuring HBV DNA to see if the patient has occult infection, which is less common (~8%).
- It may also mean the patient is in the “window phase” with a resolving an acute infection and anti-HBs hasn’t formed yet.
- A false-positive anti-HBc is also possible but rare with modern assays.
Pearl 4:
- In asymptomatic patients from endemic areas screened for HBV, a positive HBsAg implies chronic infection. IgM anti-HBc is not required to confirm chronic infection.
- Immunocompetent adults acutely infected with HBV are more likely to show symptoms and less likely to develop chronic infection, whereas young children or immunosuppressed persons are less likely to have symptoms of acute infection and more likely to develop chronic infection.
Pearl 5:
- HBeAg is a marker of active replication and increases risk of viral transmission and hepatocellular carcinoma.
- The majority of people infected with HBV perinatally will seroconvert from HBeAg positive to negative around 30 years of age. HBeAg seroconversion is associated with decrease in viral load levels and lower rates of hepatocellular carcinoma (HCC)
- A subset of HBeAg negative patients can develop mutations in the HBV genome that is integrated into the patient’s hepatocytes, leading to increasing viral replication and risk for HHC.
References
- Chou R, Dana T, Bougatsos C, Blazina I, Khangura J, Zakher B. Screening for Hepatitis B Virus Infection in Adolescents and Adults: A Systematic Review to Update the U.S. Preventive Services Task Force Recommendation. Ann Intern Med. 2014; 161:31-45. Doi: 10.7326/M13-2837
- Interpretation of Hepatitis B Serologic Test Results. Centers for Disease Control and Prevention. https://www.cdc.gov/hepatitis/hbv/pdfs/chronichepbtestingflwup.pdf.
- Abara WE, Qaseem A, Schillie S, McMahon BJ, Harris AM, . Hepatitis B Vaccination, Screening, and Linkage to Care: Best Practice Advice From the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2017;167:794–804. doi: 10.7326/M17-1106
- Recommendations for Routine Testing and Follow-up for Chronic Hepatitis B Virus (HBV) Infection. Centers for Disease Control and Prevention. https://www.cdc.gov/hepatitis/hbv/pdfs/serologicchartv8.pdf.
- Lin K and Vickery J. Screening for Hepatitis B Virus Infection in Pregnant Women: Evidence for the U.S. Preventive Services Task Force Reaffirmation Recommendation Statement. Ann Intern Med. 2009;150:874-876.
- Bersoff-Matcha SJ, Cao K, Jason M, Ajao A, Jones SC, Meyer T, Brinker A. Hepatitis B Virus Reactivation Associated With Direct-Acting Antiviral Therapy for Chronic Hepatitis C Virus: A Review of Cases Reported to the U.S. Food and Drug Administration Adverse Event Reporting System. Ann Intern Med. 2017; 166:792-798. doi:10.7326/M17-0377
- Perillo RP, Gish R, and Falck-Ytter YT. American Gastroenterological Association Institute Technical Review on Prevention and Treatment of Hepatitis B Virus Reactivation During Immunosuppressive Drug Therapy. Gastroenterology 2015; 148:221-244.
- Preexposure Prophylaxis for the Prevention of HIV Infection in the United States – 2014. A Clinical Practice Guidelines. US Public Health Service. Centers for Disease Control and Prevention.
- Viral Hepatitis. Centers for Disease Control and Prevention, 4 Aug. 2016, www.cdc.gov/hepatitis/hbv/hbvfaq.htm. Accessed January 11, 2018.
- Use of Hepatitis B Vaccination for Adults with Diabetes Mellitus: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report. Dec 23, 201160(50);1709-1711