Peer Reviewed
Abstract
Atrial fibrillation (AF) is a common arrhythmia, especially in the elderly, and is often asymptomatic. However, absence of symptoms does not confer better prognosis. Many patients with AF present with stroke as their first manifestation. In the United States, no guidelines exist to screen for AF. Given the associated morbidity of AF and significant stroke risk reduction with oral anticoagulation, this article seeks to address whether patients should be screened for AF in primary stroke prevention.
Older patients may benefit from single timepoint opportunistic pulse taking and confirmation ECG; however, the evidence is limited and must be interpreted with caution. While a significant portion of patients with screen-detected AF may qualify for anticoagulation, it is unclear which patients would benefit with improved vascular outcomes.
Introduction
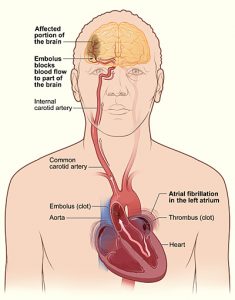
Atrial fibrillation (AF) is a sustained arrhythmia commonly encountered in clinical practice, especially in those over age 65. About a third of patients with AF are asymptomatic [1,2]. However, absence of symptoms does not confer a better prognosis and is associated with a higher incidence of cerebrovascular disease [3]. Up to 5% of patients with AF present with ischemic stroke as their first manifestation [4]. Further, AF may be newly diagnosed with prolonged cardiac monitoring modalities in nearly a quarter of patients after stroke or transient ischemic attack [5]. The American Heart Association, American College of Cardiology, and Heart Rhythm Society provide no guidelines for screening [6]. Given the associated morbidity of AF and significant stroke risk reduction with oral anticoagulation [7], should patients be screened for AF in primary stroke prevention?
Opportunistic versus systematic screening
Opportunistic screening is offered during a routine medical visit, while systematic screening involves screening the general population or high-risk groups en masse. In the British, multicenter Screening for Atrial Fibrillation in the Elderly (SAFE) study [8], opportunistic screening with pulse palpation was noninferior in AF detection to systematic population screening with electrocardiogram (ECG) in those aged 65 or older, although only about 50% of invited patients participated in systematic screening. The authors noted extensive screening coverage partly increased utility of opportunistic screening. Both single timepoint methods were more effective than routine practice with no structured approach for detection of AF. This suggests routine ECG is unnecessary in this population as long as providers are conscientious about palpating the pulse.
A recent Cochrane systematic review identified the SAFE trial as the only high-quality evidence of AF screening in the elderly. In studies considered moderate-quality evidence, both opportunistic and systematic screening had higher rates of AF detection than routine practice [9]. Taken together, the AF-SCREEN International Collaboration—a multidisciplinary group formed in 2015 to promote AF screening research for primary stroke prevention—recognizes a potential benefit to single timepoint opportunistic pulse taking with confirmatory ECG in patients aged 65 years or older based on stroke risk from a virtual CHADS2-VASc score [10].
However, single timepoint screening may be limited in detecting paroxysmal AF. The Swedish study, Population screening of 75- to 76-year-old men and women for silent atrial fibrillation (STROKESTOP) [11], examined multiple timepoint AF screening using twice daily patient-activated handheld ECGs over 2 weeks for patients aged 75 or 76. An additional 3% of patients received new AF diagnoses with multiple timepoint screening, with higher yield in those with more than one stroke risk factor. The majority of these patients accepted oral anticoagulation therapy.
Other screening methods
New technologies have expanded the range of inexpensive diagnostic cardiac monitoring tools. Finger photoplethysmography using a smartphone camera and flash has similar sensitivity and specificity for AF detection as a 12-lead ECG [8]. Low-cost, handheld, single-lead ECGs are also available, many of which can be attached to smartphones for data upload and transfer. Repeated handheld readings compare well to 12-lead tracings in diagnostic accuracy [12]. Given the wide availability of smartphones, these handheld devices are attractive tools, but require noise-free environments for optimal performance and still require confirmatory 12-lead ECG. Continuous cardiac monitoring with Holter monitors, external loop recorders, and implantable devices are more effective in AF detection than handheld devices, but patient compliance and significant costs make them impractical options [10].
Potential screening benefits and relevance of screen-detected AF
No completed randomized control trials have evaluated antithrombotic therapy specifically in screen-detected AF; however, most patients with screen-detected AF have moderate to high stroke risk based on CHADS2-VASc score, suggesting a majority would be eligible for oral anticoagulation [13]. In incidentally discovered asymptomatic AF, 1 large cohort study found that oral anticoagulation compared to no antithrombotic therapy was associated with significantly reduced adjusted risk of stroke and mortality after 1.5 years of treatment [14]. Post-hoc analysis in non-vitamin K oral anticoagulant studies reveals similar efficacy and safety of warfarin between patients with permanent and nonpermanent AF [15]. The same benefits of anticoagulation therapy may potentially apply to patients with screen-detected AF.
However, without clear evidence demonstrating AF screening can lead to better vascular outcomes, the utility of AF screening is debated. Nonpermanent forms of AF may have a quarter lower risk of stroke than permanent forms of AF [16]. Further, the threshold of AF duration or burden required to confer stroke risk and ultimately benefit from anticoagulation remains unclear. Recently, the Fibrillation reduction atrial pacing trial (ASSERT) [17] found that patients with the longest asymptomatic AF episodes (>17.72 hours) on implanted cardiac monitoring devices were at significantly increased risk of stroke. Overall, the reported increase in risk varies based on minimum length of device-detected AF (5 minutes to 24 hours) [18]; however, device-detected AF and paroxysmal AF meeting criteria in trials establishing stroke risk reduction with anticoagulation may not have similar implications [19]. Several ongoing trials seek to address the asymptomatic AF burden necessary to warrant treatment and the potential benefit of anticoagulation in subclinical AF (NCT01593553, NCT01938248, NCT02618577).
Moreover, recent arguments propose that AF may not cause ischemic stroke through atrial stasis and thrombogenesis alone, but rather may confer stroke risk via a more complex model that includes systemic vascular and atrial substrate abnormalities [20]. This model is based on observations that not all patients with AF face significant stroke risks [21], not all patients with AF have strokes with embolic features [22], some patients do not manifest AF until after stroke despite months of pre-stroke continuous heart rhythm monitoring [23], and successful rhythm-control strategies may not necessarily reduce stroke risk [24]. To address these discrepancies, the proposed model suggests aging and systemic vascular risk factors can cause atrial cardiopathy, which can manifest as AF and thromboembolism. In turn, AF can worsen atrial cardiopathy. Additionally, noncardioembolic strokes can lead to systemic inflammatory and autonomic changes that may also increase AF risk. Together, this model implies possible untested markers besides dysrhythmia detection may augment screening for stroke risk.
Conclusion
AF screening for the primary prevention of strokes may identify a significant number of patients who could qualify for anticoagulation, but it is unclear which patients would actually benefit from this intervention. Single timepoint opportunistic pulse palpation with confirmatory ECG in elderly adults is potentially feasible and beneficial for primary stroke prevention. Additional screening with twice-daily intermittent ECGs for 2 weeks may be warranted in patients older than 75 years. Given that health screening varies with resource availability, country, and healthcare system, the limited AF screening evidence must be interpreted with caution. Newer inexpensive technologies, including handheld devices, are promising tools for screening. Future research (NCT01593553, NCT01291953) may provide more data on screening and the benefits of treating asymptomatic AF, but could also examine novel mechanistic approaches to AF stroke risk, potentially augmenting screening strategies.
Dr. Dixon Yang is a resident physician, neurology at NYU Langone Health
Peer reviewed by Danielle Stember, MD, neurologist at NYU Langone Health
Image courtesy of Wikimedia Commons
References
- Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129(8):837-47. doi: 10.1161/CIRCULATIONAHA.113.005119. https://www.ncbi.nlm.nih.gov/pubmed/24345399
- Quirino G, Giammaria M, Corbucci G, et al. Diagnosis of paroxysmal atrial fibrillation in patients with implanted pacemakers: relationship to symptoms and other variables. Pacing Clin Electrophysiol. 2009;32(1):91–8.
- Flaker GC, Belew K, Beckman K, et al. Asymptomatic atrial fibrillation: demographic features and prognostic information from the Atiral Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Am Heart J. 2005;149(4):657-63. https://www.ncbi.nlm.nih.gov/pubmed/15990749
- Lubitz SA, Yin X, McManus DD, et al. Stroke as the initial manifestation of atrial fibrillation: the Framingham Heart Study. 2017;48(2):490-2.
- Sposato LA, Cipriano LE, Saposnik G, Vargas ER, Riccio PM, Hachinski V. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol. 2015;14(4):377-87.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):e199–267. https://www.ncbi.nlm.nih.gov/pubmed/24682348
- Hart RG, Pearce LA, Koudstaal PJ. Transient ischemic attacks in patients with atrial fibrillation: implications for secondary prevention: the European Atrial Fibrillation Trial and Stroke Prevention in Atrial Fibrillation III trial. 2004;35(4):948.
- Fitzmaurice DA, Hobbs FDR, Jowett S, et al. Screening versus routine practice in detection of atrial fibrillation in patients aged 65 or over: cluster randomised controlled trial. BMJ. 2007;335(7616):383. https://www.ncbi.nlm.nih.gov/pubmed/17673732
- Moran PS, Teljeur C, Ryan M, Smith SM. Systematic screening for the detection of atrial fibrillation. Cochrane Database Syst Rev. 2016;(6):CD009586. DOI: 10.1002/14651858.CD009586.pub3.
- Freedman B, Camm J, Calkins H, et al. Screening for atrial fibrillation: a Report of the AF-SCREEN International Collaboration. Circulation. 2017;135(19):1851-67. doi: 10.1161/CIRCULATIONAHA.116.026693. https://www.ncbi.nlm.nih.gov/pubmed/28483832
- Svennberg E, Engdahl J, Al-Khalili F, Friberg L, Frykman V, Rosenqvist M. Mass screening for untreated atrial fibrillation: the STROKESTOP study. Circulation. 2015;131(25):2176-84.
- McManus DD, Chong JW, Soni A, et al. PULSE-SMART: Pulse-based arrhythmia discrimination using a novel smartphone application. J Cardiovasc Electrophysiol. 2016;27(1):51-7. doi: 10.1111/jce.12842.
- Lowres N, Neubeck L, Redfern J, Freedman SB. Screening to identify unknown atrial fibrillation. A systematic review. Thromb Haemost. 2013;110(2):213-22.
- Martinez C, Katholing A, Freedman SB. Adverse prognosis of incidentally detected ambulatory atrial fibrillation: a cohort study. Thromb Haemost. 2014;112(2):276-86.
- Vanassche T, Lauw MN, Eikelboom JW, et al. Risk of ischemic stroke according to pattern of atrial fibrillation: analysis of 6563 aspirin-treated patient sin ACTIVE-A and AVERROES. Eur Heart J. 2015;36:281-7a.
- Ganesan AN, Chew CP, Hartshorne T, et al. The impact of atrial fibrilliation type on the risk of thromboembolism, mortality, and bleeding: a systematic review and meta-analysis. Eur Heart J. 2016;37:1591-602.
- Van Gelder IC, Healey JS, Crijns HJGM, et al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J. 2017;38(17):1339-44.
- Boriani G, Glotzer TV, Santini M, et al. Device-detected atrial fibrillation and the risk for stroke: analysis from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation information from implanted devices). Eur Heart J. 2014;35(8):508-16.
- Thijs V. Atrial fibrillation detection: fishing for an irregular heartbeat before and after stroke. Stroke. 2017;48:2671-7.
- Kamel H, Okin PM, Elkind MSV, Iadecola C. Atrial fibrillation and mechanisms of stroke: time for a new model. 2016;47:895-900.
- Chao TF, Liu CJ, Chen SJ, et al. Atrial fibrillation and the risk of ischemic stroke: does it still matter in patients with a CHA2DS2-VASc score of 0 or 1? Stroke. 2012;43:2551–5.
- Lodder J, Bamford JM, Sandercock PA, Jones LN, Warlow CP. Are hypertension or cardiac embolism likely causes of lacunar infarction? Stroke. 1990;21:375–81.
- Brambatti M, Connolly SJ, Gold MR, et al. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation. 2014;129:2094–9.
- Al-Khatib SM, Allen LaPointe NM, Chatterjee R, et al. Rate- and rhythm-control therapies in patients with atrial fibrillation: a systematic review. Ann Intern Med. 2014;160:760–73.