Peer Reviewed
The concept of lactate is frequently on the tip of the tongue or top of the to-do list in the hospital setting. As a new hospitalist, I am frequently discussing with house staff the clinical significance of this molecule and how to interpret its elevation.
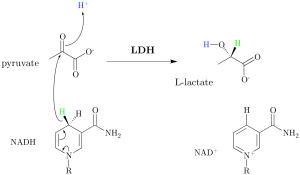
What is lactate, anyway? Lactate is a hydroxycarboxylic acid that is produced or removed by a reversible oxido-reduction reaction catalyzed by the enzyme lactate dehydrogenase (LDH), which is located in the cytosol of human cells. In humans, approximately 65% of plasma lactate is derived from glucose, and 16-20% is derived from alanine; it is the end-product of the metabolic pathways that break down these molecules. Once produced, lactate is cleared when it is oxidized in the cytosol into pyruvate, which is then shuttled to the mitochondrial matrix for further metabolism. In healthy humans, lactate doesn’t accumulate because, in the various tissues and organs (from the heart to the liver to skeletal muscle to human blood cells), all is balanced and the reversible reaction described above favors lactate clearance [1].
However, this does not always happen. Classically, we think of lactate as evidence of end-organ hypoperfusion, or shock, and lactate monitoring plays an immensely important role when it comes to prognostication and risk assessment in these situations. However, in this article, we explore how hyperlactatemia can be viewed in the context of other medical problems and the interesting alternative pathophysiology that produces lactate elevations in these settings.
Before we dive in, it is important to touch on the large body of literature that exists on how blood gases are processed. Though the jury is still out on many of the pre-analytical variables—glass vs plastic syringes, storage on ice vs at room temperature, use of a pneumatic tube system vs hand transport of samples, effect of a tourniquet—it is uncontested that cellular metabolism continues even after a blood sample is collected, and this may lead to an increase in lactate [2, 3, 4, 5]. To prevent skewing of values, guidelines state blood gasses should be analyzed within 30 minutes of collection [2].
Now, onto lactate interpretation.
In patients with asthma:
Though the association between severe acute asthma and elevated lactate has been described in the literature for decades, recent research suggests this phenomenon is much more common than initially reported. Per a 2014 review, prospective observational studies have found that between 50% and 80% of adult and child patients in the emergency room with this problem present with a lactate level above 2.2 mmol/L [6].
Multiple factors contribute to the elevated lactate seen in the setting of acute severe asthma. These include excessive muscle work, the use of beta-agonists for treatment, and the counter-regulatory response to respiratory alkalosis [7, 8, 9].
Though all organs produce lactate, muscle is the largest generator of the molecule. In asthma, as well as other conditions in which hyperventilation occurs, respiratory muscles are overworked because of increased inspiration and expiration. This excess work can contribute to the elevated lactate observed. Interestingly, in studies that looked at the association between acute severe asthma and hyperlactatemia, an inverse association between lactate and phosphate levels exists. The hypophosphatemia is believed to precede the hyperlactatemia, which likely reflects respiratory muscles undergoing an increased rate of glycolysis and using up phosphate stores [9].
In addition, many studies have confirmed a relationship between the beta-2 agonist therapy used in asthma and elevated lactate. Though the definitive mechanism is incompletely understood, many theorize that albuterol may potentiate the adrenergic state already associated with asthma. This heightened adrenergic state leads to increased lactate production through up-regulation of glycolysis and increased pyruvate production, increased lipolysis-mediated inhibition of pyruvate dehydrogenase, and stimulation of B2-receptors and subsequent increases in substrate for glycolysis [10]. Though exceptions exist, the hyperlactatemia associated with treatment of asthma is usually not accompanied by a metabolic acidosis, and the observed lactate elevation in this condition is not associated with worse outcomes [6, 8].
In patients with liver dysfunction:
The liver is the primary organ responsible for lactate clearance, and liver dysfunction can disrupt this function [7]. Much of the literature on this subject focuses on hepatic dysfunction in patients with sepsis, frequently in those who are critically ill. A study published in 1999 looked at patients with acute circulatory failure and the effect of hepatic dysfunction on lactate levels. Though there were no differences in other variables including lowest systolic blood pressure, serum creatinine, and simplified acute physiology score, lactate levels were significantly higher in the group with liver disease (8.24 mmol/L vs 4.29 mmol/L; P < 0.001), and a positive correlation was found between lactate and AST levels (R = 0.55; P < .001) [11]. Given no difference in the clinical parameters noted above, the authors ruled out a correlation between liver dysfunction and severity of shock as a possible confounder their results.
In a 2015 study of 187 patients undergoing early sepsis resuscitation, researchers studied the effect of liver dysfunction on lactate normalization. The patients were stratified based on the extent of liver disease (no liver disease, mild liver disease, or cirrhosis). The authors noted cirrhotic patients had higher baseline lactate levels and that advanced liver disease was associated with impaired lactate clearance and normalization [12]. Of note, this did not translate into a statistically significant difference in mortality.
With regard to patients with chronic liver disease and without sepsis, 1 study published in 2013 involved hepatic vein catheterization in patients with and without chronic liver disease and blood samples before and after stimulation with galactose. In this study, the average fasting lactate concentration in the hepatic vein was 0.92 in male patients and 0.93 in female patients with liver disease, and 0.32 in male patients and 0.52 in female patients without liver disease (P < 0.001) [13]. However, a handful of older articles studying lactate levels in non-septic patients do not show as significant of a correlation between hepatic dysfunction and lactate levels. In a study published in 1987 that looked at critically ill patients with severe liver disease, Kruse et al only found elevated lactate levels in patients with clinical evidence of circulatory shock [14]. Woll et al studied lactate clearances in patients with cirrhosis and healthy volunteers following lactate infusions; though they found a significantly prolonged half-life of lactate elimination in the cirrhotic patients, there was no elevation in the fasting basal lactate levels in the cirrhotic group [15].
Taken together, it appears that when lactate levels are elevated for another reason, liver dysfunction inhibits its clearance; however, it is unclear if hyperlactatemia can be solely attributed to liver dysfunction.
In patients with kidney disease:
Following the liver, the kidney is believed to be the major lactate-consuming organ in the body, and thus, kidney function should be taken into account when investigating an elevated lactate value [16]. In fact, in a letter to the editor responding to the 1987 Kruse article described above, Guerin and colleagues wrote that, since renal insufficiency leads to lactate accumulation, differences in kidney function between the two described groups might have confounded the findings [17].
In exogenous lactate infusion studies in rats, dogs, and sheep, the kidneys have been found to be responsible for the removal of 20-30% of an exogenous load, mostly via metabolism but also via excretion at higher lactate loads. In nephrectomy studies in the same cluster of mammals, the half-life for lactate elimination increases from 5.3 to 7.1 minutes after nephrectomy was performed [16].
As with the literature surrounding lactate and the liver, much of the research on kidney disease and lactate describes patients with sepsis. In a recently published abstract, it was determined that, of 3786 patients with severe sepsis or septic shock, patients with acute kidney injury had a lower median lactate clearance at 3 hours and 6 hours compared to those without acute kidney injury (P < 0.001) [18].
In patients with diabetes (on metformin):
Lactic acidosis is an often-cited adverse effect of metformin therapy, though it was much more common occurrence with earlier generations of bigaunides which are no longer in use. The mechanism of this side effect is complex but, in part, involves the inhibition of oxidative phosphorylation in the mitochondria and aerobic respiration. The earlier bigaunides are more lipid soluble than metformin and can more readily cross the mitochondrial membrane, and thus, more meaningfully cause this problem [19].
There are two proposed types of lactic acidosis related to metformin therapy: metformin-associated lactic acidosis (MALA) and metformin-induced lactic acidosis (MILA) [20]. In MALA, the lactic acidosis is caused by metformin accumulation in the setting of a precipitating factor, such as sepsis, dehydration, or kidney disease. In MILA, metformin is the only apparent cause of the lactic acidosis.
Importantly, studies have explored the association between metformin and the development of lactic acidosis in hospitalized patients and have found that though difficult to exclude the role of metformin, patients with lactic acidosis have an alternative etiology of the lactate elevation, hence MALA is a much more common entity than MILA [21]. Comprehensively, a large Cochrane meta-analysis from 2010 pooled data from 347 comparative trials and cohort studies involving type 2 diabetic patients on metformin and other anti-hyperglycemic treatments. The study found that no cases of fatal or non-fatal lactic acidosis occurred in 70,490 patient-years of metformin use or in 55,451 patient-years in non-metformin group, and the incidence of lactic acidosis per 100,000 patient-years was 4.3 cases in the metformin group and 5.4 in the non-metformin group [22].
Overall, MILA is an entity that can exist in the setting of metformin overdose or when metformin accumulates secondary to a concomitant condition, such as kidney failure. Otherwise, hyperlactatemia should not be attributed solely to metformin therapy, and other etiologies should be explored. Notably, in DKA elevated lactate levels are also found, often secondary to hypovolemia or accumulation of D-lactate.
In patients with alcoholism:
Lactic acidosis can develop in patients with both acute alcohol intoxication as well as chronic use. On a cellular level, the oxidation of ethanol decreases the NAD+/NADH ratio, which subsequently shifts pyruvate towards lactate. In 2005, Zehtabchi et al published a study aimed to answer whether ethanol can explain the acidosis commonly seen in ethanol-intoxicated patients. The study found that ethanol intoxication was associated with a higher lactate level (mean difference: 0.69 mmol/L; 95% CI, 0.11 to 1.27 mmol/L) [23]. Further, studies with healthy volunteers have also shown a predictable rise in plasma lactate after ethanol ingestion. Still, given the modest increase in lactate ethanol causes and the frequent co-morbidities that exist with alcoholism, it is important to rule out other causes of elevated lactate when hyperlactatemia is seen in hospitalized patients with alcohol use.
Though it is imperative to not lose sight of the relationship between lactate and hypoperfusion, the above highlights some of the prevalent conditions seen in the hospital setting which may contribute to an observed elevated lactate. The list is incomplete, as additional causes of lactate elevation, including seizures and malignancy, are also common. Further, with all of this said, practitioners should also be aware of an opposing phenomenon—an entity in which sepsis-induced hypotension is observed in the absence of lactatemia [24]. Overall, lactate values should not be interpreted in a bubble, and if a lactate value is obtained, its thoughtful interpretation is always indicated.
Dr. Rebecca Lazarus is a hospitalist at Bellevue Hospital and instructor in clinical medicine at NYU Langone Health.
Peer reviewed by Harald Sauthoff, MD, critical care, pulmonary medicine, NYU Langone Health
Image courtesy of Wikimedia Commons
References
1) Adeva-Andany M, López-Ojén M, Funcasta-Caderón R, Ameneiros-Rodriguez E, et al. Comprehensive review on lactate metabolism in human health. Mitochondrion. 2014;17:76-100. https://www.ncbi.nlm.nih.gov/pubmed/24929216
2) Dukić L, Kopčinović LM, Dorotić A, Baršić I. Blood gas testing and related measurements: National recommendations on behalf of the Croatian Society of Medical Biochemistry and Laboratory Medicine. Biochem Med (Zagreb). 2016;23(3):318-336. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5082214/
3) Baird G. Preanalytical considerations in blood gas analysis. Biochem Med (Zagreb). 2013;23(1):19-27. https://www.ncbi.nlm.nih.gov/pubmed/23457763
4) Collinson PO, John CM, Gaze DC, Ferrigan LF, Cramp DG. Changes in blood gas samples produced by a pneumatic tube system. J. Clin. Pathol. 2002;55(2):105-107. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1769597/
5) Balakrishnan V, Wilson J, Taggart B, Cipolla J, Jeanmonod R. Impact of phlebotomy tourniquet use on blood lactate levels in acutely ill patients. CJEM. 2016;18(5):358-362. https://www.ncbi.nlm.nih.gov/pubmed/27618976
6) Rodrigo GJ. Serum lactate increase during acute asthma treatment: A new piece of the puzzle Chest. 2014;145(1):6-7. https://www.ncbi.nlm.nih.gov/pubmed/24394811
7) Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. Etiology and therapeutic approach to elevated lactate. Mayo Clin Proc. 2013;88(10):1127-1140. https://www.ncbi.nlm.nih.gov/pubmed/24079682
8) Lewis LM, Ferguson I, House SL, et al. Albuterol administration is commonly associated with increases in serum lactate in patients with asthma treated for acute exacerbation of asthma. Chest. 2014;145(1):53-59. https://www.ncbi.nlm.nih.gov/pubmed/23949578
9) Rabbat A, Laaban JP, Boussairi A, Rochemaure J. Hyperlactatemia during acute severe asthma. Intensive Care Med. 1998;24(4):304-312. https://www.ncbi.nlm.nih.gov/pubmed/9609407
10) Pourmand A, Dorwart K, Mazer-Amirshahi M, Nasser S, Shokoohi H. Beta agonist-induced lactic acidosis, an evidence-based approach to a critical question. Am J Emerg Med. 2016;34(3):666-668. https://www.ncbi.nlm.nih.gov/pubmed/26830219
11) DeJonghe B, Cheval C, Misset B, et al. Relationship between blood lactate and early hepatic dysfunction in acute circulatory failure. J Crit Care. 1999;14(1):7-11. https://www.ncbi.nlm.nih.gov/pubmed/10102718
12) Sterling SA, Puskarich MA, Jones AE. The effect of liver disease on lactate normalization in severe sepsis and septic shock: a cohort study. Clin Exp Emerg Med. 2015;2(4): 197-202. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5052907/
13) Jeppesen JB, Mortensen C, Bendtsen F, Møller S. Lactate metabolism in chronic liver disease. Scand J Clin Lab Invest. 2013;73(4):293-299. https://www.ncbi.nlm.nih.gov/pubmed/23514017
14) Kruse JA, Zaidi SA, Carlson RW. Significance of blood lactate levels in critically ill patients with liver disease. Am J Med. 1987;83(1):77-82. https://www.ncbi.nlm.nih.gov/pubmed/3605185
15) Woll PJ, Record CO. Lactate elimination in man: effects of lactate concentration and hepatic dysfunction. Eur J Clin Invest. 1979;9(5):397-404. https://www.ncbi.nlm.nih.gov/pubmed/118034
16) Bellomo R. Bench-to-bedside review: lactate and the kidney. Crit Care. 2002;6(4):322-326. https://www.ncbi.nlm.nih.gov/pubmed/12225607
17) Guerin JM, Meyer P, Segregtaa JM. Is blood lactate determination really reliable and useful in critically ill patients with liver disease? Am J Med. 1988;84(2):374. https://www.ncbi.nlm.nih.gov/pubmed/3407671
18) Taylor B, Register L, Karvestski C, Taylor S. The effect of lactate clearance on mortality in patients with acute kidney injury. Crit Care Med. 2016;44(12):435 (Abstract).
19) Weisberg L. Lactic acidosis in a patient with type 2 diabetes mellitus. Clin J Am Soc Nephrol. 2015;10(8):1476-1483. https://www.ncbi.nlm.nih.gov/pubmed/25762524
20) Visconti L, Ferrara D, Costantino G, Aloisi C, et al. Metformin-related lactic acidosis: is it a myth or an underestimated reality? Ren. Fail. 2015;38(9):1560-1565. https://www.ncbi.nlm.nih.gov/pubmed/27686366
21) Inzucchi SE, Lipska KJ, Mayo H, et al. Metformin in patients with type 2 diabetes and kidney disease. JAMA. 2014;312(24):2668-2675. https://www.ncbi.nlm.nih.gov/pubmed/25536258
22) Salpeter SR, Greyber E, Pasternak GA, Selpeter EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus Cochrane Database Syst Rev. 2010;20(1). https://www.ncbi.nlm.nih.gov/pubmed/20091535
23) Zehtabchi S, Sinert R, Baron BJ, Paladiono L, et al. Does ethanol explain the acidosis commonly see in ethanol-intoxicated patients? Clin Toxicol (Philla). 2005;43(3):161-166. https://www.ncbi.nlm.nih.gov/pubmed/15902789
24) Hernandez G, Bruhn A, Castro R, et al. Persistent sepsis-induced hypotension without hyperlactatemia: A distinct clinical and physiological profile within the spectrum of septic shock. Crit Care Res Pract. Epub 2012 April 18. https://www.ncbi.nlm.nih.gov/pubmed/22570774