Peer Reviewed
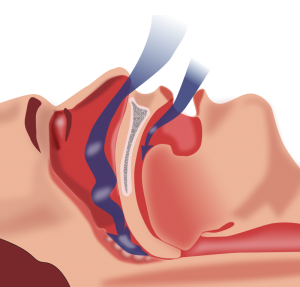
Obstructive sleep apnea (OSA) is an increasingly prevalent disorder that has well described associations with cardiovascular disease. OSA affects approximately 20–30% of males and 10–15% of females in North America.1-3 In addition to male gender, other risk factors for OSA include obesity, older age, and hypertension.4 The pathophysiology of OSA hinges on increased upper airways resistance and the collapse of the upper airway during sleep.5 This causes partial to complete obstruction of airflow, leading to hypopnea, apnea, and disturbances of sleep. OSA can be diagnosed in patients with 5 or more apnea or hypopnea events per hour of sleep.4 Focuses on the relief of upper airway obstruction during sleep, treatment of OSA includes weight loss, surgeries to reduce the burden of excess upper airway tissue, mandibular advancement devices, and continuous positive airway pressure (CPAP). CPAP acts as a pneumatic splint to open the upper airway during sleep, preventing airway collapse and consequent arousals provoked by interrupted airflow. CPAP is generally considered first line therapy for severe OSA.6
Untreated OSA has been associated with increased risk of cardiovascular morbidity and mortality.7 Numerous physiologic determinants have been implicated in this association, some of which will be reviewed presently. Arousals from sleep, hypoxia, and occasionally hypercapnia lead to increased sympathetic nervous system activation, causing peripheral vasoconstriction and elevated blood pressure.8,9 Negative intrathoracic pressure generated against an obstructed airway, in addition to the aforementioned hypoxia and hypercapnia, may lead to increased right and left ventricular afterload, and increased myocardial oxygen demand coupled with decreased oxygen delivery. Sustained oxygen desaturation associated with hypopneas and apneas can lead to hypoxic pulmonary vasoconstriction, resulting in increased pulmonary artery pressures.4 The pathogenesis of cardiovascular disease in patients with OSA is further substantiated by the wealth of literature linking OSA to specific ailments. Approximately 20% of patients with moderate to severe OSA develop pulmonary hypertension.10 Patients with OSA have a 3 to 5 times greater risk of developing atrial fibrillation than the general population.11,12 Studies have suggested that OSA may be a risk factor for the development of heart failure, stroke, and worsening coronary artery disease.13-15 These conditions are consequences of increased sympathetic tone occurring due to arousals from sleep.
Hypertension is among the most abundantly described comorbidities linked to OSA. Some data suggest a correlation between the severity of OSA and the risk of developing hypertension; as the number of obstructive events per hour of sleep increase, risk approaches 2 to 3 times that of the general population.16 This relationship prompts a compelling question: does reducing the severity of OSA correspond to an improvement in the associated comorbidities? Randomized controlled trials have shown that CPAP has modest effects on blood pressure, with decreases in systolic blood pressure by 2–3 mm Hg in normotensive patients, and 6–7 mm Hg in patients with resistant hypertension.17,18 However, the larger question of whether or not CPAP reduces the risk of cardiovascular morbidity and mortality has proved more challenging.
Observational studies have demonstrated that among patients with OSA, CPAP is associated with a lower incidence of fatal and nonfatal cardiovascular events.7,19 A recent meta-analysis of observational studies corroborated these findings, noting a hazard ratio (HR) of 0.37 (95% CI, 0.16 to 0.54) for cardiovascular mortality in CPAP treated patients compared to untreated patients.20 However, these studies are marred by their lack of randomization. Therefore, the patients compliant with CPAP may have enjoyed their cardiovascular benefit from any number of downstream effects of their general aptitude towards making healthy lifestyle choices (the healthy user bias) rather than from CPAP alone.
The positive results from these observational studies were not borne out in larger randomized controlled trials (RCTs). A multicenter study out of Spain randomized 725 patients with moderate to severe OSA (but no daytime sleepiness or history of cardiovascular disease) to receive either CPAP or no intervention.21 After a median follow up of 4 years, the trial demonstrated that there was no significant difference in cardiovascular events between groups. Only a post hoc analysis of patients who used CPAP for 4 hours per night or longer suggested.22 that CPAP was associated with a reduction in the incidence of adverse cardiovascular outcomes A smaller secondary prevention RCT involving 224 patients with OSA and coronary artery disease similarly showed no difference in cardiovascular endpoints in patients receiving CPAP. However, an adjusted on-treatment analysis showed significant cardiovascular risk reduction in the patients who used CPAP for more than 4 hours per night (HR, 0.29; 95% CI, 0.10 to 0.86).23 The benefits of CPAP demonstrated by the post hoc analyses of these 2 studies, while encouraging, must be interpreted with caution as they are based on nonrandomized comparisons and may be influenced by similar biases discussed in the aforementioned observational studies.
The Sleep Apnea Cardiovascular Endpoints (SAVE) trial was the largest RCT evaluating CPAP for prevention of cardiovascular events in OSA.24 The SAVE trial was a multicenter, open label, secondary prevention study that randomized 2717 patients with moderate to severe OSA and coronary or cerebrovascular disease into 2 groups: CPAP plus usual care or usual care alone. The primary end point was a composite of acute coronary syndrome, stroke or transient ischemic attack, hospitalization for heart failure, and death from any cardiovascular cause.24 After a mean follow up of 3.7 years the primary end point event occurred in 17% of the patients in the CPAP group and in 15.4% in the usual care group (P = .34). CPAP significantly reduced daytime sleepiness, snoring, and the number of hourly apnea and hypopnea events by nearly 90%. However, CPAP did not significantly affect any individual or composite cardiovascular outcome. While this may be the most robust RCT evaluating CPAP for the prevention of cardiovascular events, there are a number of limitations. First and foremost, the mean adherence to therapy in the CPAP group was 3.3 hours per night; while this is consistent with typical use, prior data suggests the greatest cardiovascular benefit in patients with at least 4 hours of use per night. Secondly, with a follow up of only 3.7 years; long-term cardiovascular benefits may have gone undetected. Lastly, the trial excluded patients with severe sleepiness, a population of patients that may be most at risk for developing cardiovascular complications.25
The SAVE trial was incorporated in 2 recent meta-analyses that sought to answer the question of whether CPAP reduces cardiovascular events in patients with OSA. The larger meta-analysis included 10 RCTs with a total of 7266 patients and concluded that use of positive airway pressure was not associated with decreased risk of major adverse cardiovascular events.26 However, consistent with prior data, subgroup analysis showed a statistically significant reduction in the risk of cardiovascular events in patients who had at least 4 hours CPAP per night (RR, 0.58; 95% CI, 0.34 to 0.99). A smaller meta-analysis included 4 RCTs with a total of 3780 patients, and ultimately arrived at similar results: CPAP was not associated with reduced risk of adverse cardiovascular events, with the exception of the subgroup that wore CPAP for over 4 hours per night (RR, 0.70; 95% CI, 0.52 to 0.94).22
Recurrent patterns emerge from these data reviewed here. Typical use of CPAP does not ameliorate the risks of fatal and nonfatal cardiovascular events in patients with OSA, though it may reduce symptoms of daytime sleepiness and snoring. Subgroup analyses of patients wearing CPAP over 4 hours per night suggest that CPAP may lower cardiovascular events; however, these findings are subject to significant bias. Future trials should incorporate new modalities that better facilitate CPAP adherence to further delineate cardiovascular benefit.
Commentary by Dr. Nishay Chitkara:
While upon first assessment the studies discussed in this review suggest a lack of reduction in cardiovascular events with use of CPAP, the greater message lies in the subgroup analyses. Use of CPAP for at least 4 hours per night is associated with a significant reduction in adverse cardiovascular events. Indeed, CPAP use for less than 4 hours per night leads to a lack of sufficient consolidation of sleep. Residual sleep disruption due to apneas and hypopneas with their associated arousals causes increased sympathetic tone and a sustained risk for hypertension, cardiac arrhythmias, and adverse cardiovascular events—particularly in patients with severe OSA. A full night’s use of CPAP is necessary to consolidate sleep architecture and allow adequate time for all sleep stages, particularly the restorative stages of REM sleep and slow wave sleep. Adherence to CPAP is generally defined as use of CPAP for at least 4 hours per night, for 70% of the time, because using CPAP for 4 or more hours enables patients to experience improvements in daytime somnolence, neurocognitive function, and quality of life.
As multiple studies have shown, treatment of OSA with CPAP has numerous cardiovascular benefits, including arrhythmia control and prevention of recurrence, improved glycemic control, and reduction of the risk for stroke and MI. Adherence to CPAP validates these outcomes. Although patients often struggle with adherence to CPAP, numerous methods can be used to enhance and encourage their usage, including daytime acclimatization sessions, humidification systems, and patient education. It is also helpful to determine and manage patient-specific barriers to use. For those who are ultimately unable to accommodate to CPAP, alternate effective treatments, such as oral appliance therapy, may prove beneficial.
Dr. Gregory Rubinfeld is an internal medicine resident at NYU Langone Health
Peer reviewed by Nishay Chitkara, MD, pulmonary medicine, NYU Langone Health
Image courtesy of Wikimedia.com
References:
- Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177:1006. https://www.ncbi.nlm.nih.gov/pubmed/23589584
- Young T, Palta M, Dempsey J, et al. Burden of sleep apnea: rationale, design, and major findings of the Wisconsin Sleep Cohort study. WMJ 2009; 108:246.
- Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med 2001;163:608-613.
- Basner RC. Continuous positive airway pressure for obstructive sleep apnea. N Engl J Med. 2007;356:1751–8. doi: 10.1056/NEJMct066953.
- White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med 2005;172:1363-1370.
- Loube DI, Gay PC, Strohl KP, Pack AI, White DP, Collop NA. Indications for positive airway pressure treatment of adult obstructive sleep apnea patients: a consensus statment. Chest 1999;115:863-866. https://www.ncbi.nlm.nih.gov/pubmed/10084504
- Marin JM, Carrizo SJ, Vicente E, Agusti AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365:1046-1053.
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995;96:1897-1904. https://www.ncbi.nlm.nih.gov/pubmed/7560081
- Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA 2012;307:2169-2176.
- Sanner BM, Doberauer C, Konermann M, et al. Pulmonary hypertension in patients with obstructive sleep apnea syndrome. Arch Intern Med 1997; 157:2483.
- Guilleminault C, Connolly SJ, Winkle RA. Cardiac arrhythmia and conduction disturbances during sleep in 400 patients with sleep apnea syndrome. Am J Cardiol 1983; 52:490. https://www.ajconline.org/article/S0002-9149(11)02005-4/fulltext
- Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med 2006; 173:910.
- Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation 2010; 122:352.
- Yumino D, Tsurumi Y, Takagi A, et al. Impact of obstructive sleep apnea on clinical and angiographic outcomes following percutaneous coronary intervention in patients with acute coronary syndrome. Am J Cardiol 2007; 99:26.
- Loke YK, Brown JW, Kwok CS, Niruban A, Myint PK. Association of obstructive sleep apnea with risk of serious cardiovascular events: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 2012;5:720-728.
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000; 342:1378.
- Montesi SB, Edwards BA, Malhotra A, Bakker JP. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Clin Sleep Med 2012;8:587-596
- Schwarz EI, Puhan MA, Schlatzer C, Stradling JR, Kohler M. Effect of CPAP therapy on endothelial function in obstructive sleep apnoea: a systematic review and meta-analysis. Respirology 2015;20:889-895.
- Buchner NJ, Sanner BM, Borgel J, Rump LC. Continuous positive airway pressure treatment of mild to moderate obstructive sleep apnea reduces cardiovascular risk. Am J Respir Crit Care Med 2007; 176:1274.
- Fu Y, Xia Y, Yi H, Xu H, Guan J, Yin S. Meta-analysis of all-cause and cardiovascular mortality in obstructive sleep apnea with or without continuous positive airway pressure treatment. Sleep Breath. 2017;21(1):181–189.
- Barbé F, Durán-Cantolla J, Sánchez-de-la-Torre M, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA 2012;307:2161-2168. https://www.ncbi.nlm.nih.gov/pubmed/22618923
- Abuzaid AS, Al Ashry HS, Elbadawi A, et al. Meta-analysis of cardiovascular outcomes with continuous positive airway pressure therapy in patients with obstructive sleep apnea. Am J Cardiol. 2017;120(4):693-699.
- Peker Y, Glantz H, Eulenburg C, Wegscheider K, Herlitz J, Thunström E. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with non-sleepy obstructive sleep apnea: the RICCADSA randomized controlled trial. Am J Respir Crit Care Med 2016 February 25.
- McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919-931. https://www.nejm.org/doi/full/10.1056/NEJMoa1606599
- Javaheri S, Barbe F, Campos-Rodriguez F, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol. 2017;69(7):841-858.
- Yu J, Zhou Z, McEvoy RD, et al. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta-analysis. JAMA. 2017;318(2):156-166