Peer Reviewed
The American Diabetes Association (ADA) recognizes four categories of diabetes: type 1 (T1DM), type 2 (T2DM), specific types due to other causes (including monogenic diabetes syndromes, diseases of the exocrine pancreas, and drug- or chemical-induced diabetes), and gestational.1 In recent years, diabetes secondary to pancreatic disease has garnered academic interest due to its lack of recognition in clinical practice, and it is now featured prominently in the 2020 and 2021 editions of the ADA’s Standards of Medical Care in Diabetes. Currently categorized as “Pancreatic Diabetes or Diabetes in the Context of Disease of the Exocrine Pancreas,” this condition has also been referred to as pancreatogenic diabetes and type 3c diabetes. Hereafter referred to as pancreatic diabetes, this condition is defined as the structural and functional loss of insulin secretion that occurs in the context of pancreatic dysfunction.1 Although pancreatic diabetes is most commonly the result of chronic pancreatitis, other etiologies include pancreatic carcinoma, acute pancreatitis, trauma, pancreatectomy, cystic fibrosis, hemochromatosis, and fibrocalculous pancreatopathy.1,2
If you have never heard of “pancreatic diabetes” as a diagnostic category, you are not alone. Historically, pancreatic diabetes has been considered uncommon and was estimated to account for only 1-2% of patients with diabetes in North America.3 In fact, in the ADA’s Standards from 2015 to 2019, pancreatic diabetes was relegated to the list of types of diabetes due to other causes, and readers who wished to learn more were referred back to the 2014 publication.4,5 Researchers began to question whether pancreatic diabetes was uncommon or if it was under-recognized. In 2017, a retrospective cohort study assessed over 500 cases that qualified as pancreatic diabetes based on ADA guidelines and found that clinicians properly identified these cases as pancreatic diabetes less than 3% of the time; 90% of these cases were classified as T2DM.6 As a result of studies such as this one, the ADA has called attention to the frequent misdiagnosis of pancreatic diabetes as T2DM in the very first sentence of its updated definition.1
Despite the lack of clinical recognition, pancreatic diabetes has been identified as a major contributor to cases of adult-onset diabetes. A literature review published in 2011 concluded that the prior estimates of the prevalence of pancreatic diabetes were spuriously low due to limitations in screening and imaging, and asserted that this etiology actually accounts for 5-10% of cases in patients with diabetes in Western populations.3 The following year, investigators at a major hospital in Germany used ADA guidelines to evaluate nearly 2000 admitted patients diagnosed with diabetes and found that 9.2% of these cases should be classified as pancreatic diabetes.7 However, the proportion of adults with pancreatic diabetes may be lower in the community setting. A recent study found that 1.8% of adults with diabetes in the UK met the criteria for pancreatic diabetes. Although this is significantly lower than the estimated prevalence in hospitalized patients, it is higher than the 1.1% of patients in this population who met the criteria for T1DM.6 Although estimates of disease prevalence vary based on the population studied, it is apparent that pancreatic diabetes is a much more significant diagnostic category than previously believed.
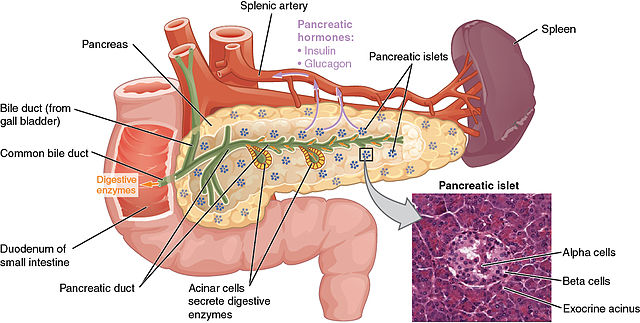
Pancreatic diabetes is characterized by varying degrees of exocrine and endocrine insufficiency and has distinct features which help distinguish it from other types. Exocrine insufficiency refers to the loss of digestive enzymes (lipase, amylase, protease, etc.) secreted through the pancreatic duct into the duodenum. While exocrine insufficiency is a defining feature of pancreatic diabetes, a reduction has also been observed in patients with T1DM and T2DM.12
Endocrine insufficiency in pancreatic diabetes refers to the loss of insulin-secreting beta cells, glucagon-secreting alpha cells, somatostatin-secreting delta cells, and pancreatic polypeptide-secreting cells, which secrete their products directly into the bloodstream.12 The loss of these hormones differs from the isolated destruction of beta cells characteristic of T1DM and the insulin resistance characteristic of T2DM. One proposed set of diagnostic criteria for pancreatic diabetes includes the following:
1) The diagnosis of DM
2) Evidence of pancreatic insufficiency (fecal elastase-1 < 200 mcg/g or abnormal direct function testing—stimulation with cholecystokinin or secretin with subsequent collection and analysis of duodenal fluid)
3) Abnormal pancreatic imaging (endoscopic ultrasound, MRI, or CT)
4) Absence of T1DM-associated autoimmune markers–most commonly antibodies to glutamic acid decarboxylase (GAD65), islet cell antibodies (ICA), insulin autoantibodies (IAA), protein tyrosine phosphatase antibodies (IA2As), and zinc transporter protein (ZnT8).2,11,12
Recently, reduced pancreatic polypeptide response to mixed nutrients has been identified as a specific marker and may help to confirm the diagnosis of pancreatic diabetes.12 Interestingly, the current ADA guidelines fail to acknowledge that T1DM and T2DM are associated with pancreatic dysfunction and that T2DM is a risk factor for pancreatic cancer.8 Therefore, pancreatic diabetes may occur in patients who have previously been diagnosed with another type and this diagnostic ambiguity should be explicitly addressed in future guidelines.
Management of pancreatic diabetes should address exocrine insufficiency. Patients with mild insufficiency may be asymptomatic or have mild abdominal discomfort, which may progress to overt steatorrhea in severe cases.12 Patients with pancreatic diabetes are at risk for deficiencies in the fat-soluble vitamins A, D, E, and K, and vitamin B12; since these deficiencies may be asymptomatic, screening is necessary. Patients may also benefit from the use of pancreatic enzyme replacement therapy (lipase, protease, and amylase) to avoid the malabsorption of fat-soluble vitamins, improve stool consistency, and improve digestion.8,9,12 Additionally, lifestyle modifications, including smoking cessation and abstaining from alcohol consumption, can prevent further pancreatic injury.8,9 Evaluating and addressing pancreatic insufficiency is required to avoid metabolic disturbance, ensure adequate nutrition, and improve the quality of life in patients with this condition.
Pancreatic diabetes has been associated with poor glycemic control and the need for early insulin therapy.1,3,8,12 The hyperglycemia in patients with pancreatic diabetes is likely exacerbated by the reduction in pancreatic polypeptide, which helps potentiate the effect of insulin on hepatic glucose metabolism, and its deficiency results in increased hepatic insulin resistance.3,12 Further research is required before evidence-based recommendations for the pharmacological management of pancreatic diabetes are available.2,3 One exception to this is the ADA recommendation for islet cell autotransplantation in patients undergoing pancreatectomy, with the goal of reducing or eliminating the need for insulin.1 Treatment with metformin is commonly considered in patients with pancreatic diabetes because it has been shown to reduce the risk of pancreatic ductal adenocarcinoma. Treatment with injectable GLP-1 analogues and oral dipeptidyl peptidase-4 inhibitors is controversial due to their association with pancreatic dysfunction.12 Another concern is the development of hypoglycemia, possibly due to the combination of low glucagon secretion and intact peripheral insulin sensitivity.12 Prior studies have suggested that up to 25% of patients with pancreatic diabetes may develop “brittle diabetes,” a term to describe severe swings in blood glucose.2,9,12 However, the development of brittle diabetes has not been observed in recent studies, may be dependent on etiology, and therefore requires further exploration.2
Awareness of pancreatic diabetes has the potential to improve the treatment and management of diabetes in the years to come.
Marie T. Mazzeo is a 2nd year student at NYU Grossman School of Medicine
Peer reviewed by Michael Tanner, MD, associate editor, Clinical Correlations
Image courtesy of Wikimedia Commons, source: http://cnx.org/content/col11496/1.6/, Jun 19, 2013.
References
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S15-S33. doi:10.2337/dc21-S002 https://pubmed.ncbi.nlm.nih.gov/33298413/
- Ewald N, Bretzel RG. Diabetes mellitus secondary to pancreatic diseases (Type 3c)–are we neglecting an important disease? Eur J Intern Med. 2013;24(3):203-206. doi:10.1016/j.ejim.2012.12.017
- Cui Y, Andersen DK. Pancreatogenic diabetes: special considerations for management. Pancreatology. 2011;11(3):279-294. doi:10.1159/000329188 https://pubmed.ncbi.nlm.nih.gov/21757968/
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81-S90. doi:10.2337/dc14-S081
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes–2019. Diabetes Care. 2019;42(Suppl 1):S13-S28. doi:10.2337/dc19-S002
- Woodmansey C, McGovern AP, McCullough KA, et al. Incidence, demographics, and clinical characteristics of diabetes of the exocrine pancreas (type 3c): a retrospective cohort study. Diabetes Care. 2017;40(11):1486-1493. doi:10.2337/dc17-0542 https://pubmed.ncbi.nlm.nih.gov/28860126/
- Ewald N, Kaufmann C, Raspe A, Kloer HU, Bretzel RG, Hardt PD. Prevalence of diabetes mellitus secondary to pancreatic diseases (Type 3c). Diabetes Metab Res Rev. 2012;28(4):338-342. doi: 10.1002/dmrr.2260. PMID: 22121010.
- Andersen DK, Korc M, Petersen GM, et al. Diabetes, pancreatogenic diabetes, and pancreatic cancer. Diabetes. 2017;66(5):1103-1110. doi: 10.2337/db16-1477. PMID: 28507210; PMCID: PMC5399609.
- Makuc J. Management of pancreatogenic diabetes: challenges and solutions. Diabetes Metab Syndr Obes. 2016;9:311-315. doi:10.2147/DMSO.S99701
- Duggan S, Ewald N, Kelleher L, et al. The nutritional management of type 3c (pancreatogenic) diabetes in chronic pancreatitis. Eur J Clin Nutr. 2017;71(1)3–8. doi.org/10.1038/ejcn.2016.127
- Chiang JL, Kirkman MS, Laffel LM, Peters AL; Type 1 Diabetes Sourcebook Authors. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care. 2014;37(7):2034-2054. doi:10.2337/dc14-1140
- Wynne K, Devereaux B, Dornhorst A. Diabetes of the exocrine pancreas. J Gastroenterol Hepatol. 2019;34(2):346-354. doi:10.1111/jgh.14451