By Sohail Zahid MD, Ph.D and Waqas Haque MD
By Sohail Zahid MD, Ph.D and Waqas Haque MD
Peer Reviewed
Clinical Case
An elderly gentleman with a medical history of coronary artery disease, atrial fibrillation, stage III chronic kidney disease, insulin-dependent type 2 diabetes mellitus, and heart failure with preserved ejection fraction (HFpEF) presented to the hospital with weeks of worsening dyspnea with exertion and progressive weakness to the extent that he was feeling significant fatigue when dressing. During this time, he denied any fevers, night sweats, chills, chest pain, orthopnea, abdominal pain, or lower extremity edema.
On admission, his initial vital signs showed a temperature of 97o F, heart rate of 98 bpm, blood pressure of 146/98 mmHg, oxygen saturation of 97% on 2 L/min nasal cannula with frequent desaturations to the mid-80s during conversation, and weight of 135 kg pounds up from his baseline of 127 kg (BMI 40 kg/m2). His physical examination showed an obese man in no acute distress, no visualizable jugular vein pulsations, irregular rate, normal S1 and S2 heart sounds without murmurs, gallops, or rubs, diminished breath sounds bilaterally without crackles or wheezes, soft adipose belly without tenderness or gross distension, mildly cool extremities, faint pulses, and trace edema.
His laboratory studies were significant for a hemoglobin of 10.0 g/dL, blood urea nitrogen of 56 mg/dL, creatinine of 2.21 mg/dL (at baseline), peak troponin I of 0.45 ng/mL (ref < 0.04 ng/mL), venous blood gas with pH of 7.39 and pCO2 of 57 mmHg (both at baseline), and B-natriuretic peptide of 627 pg/mL up from 531 pg/mL one year ago.
His chest x-ray demonstrated an enlarged cardiac silhouette and atelectasis at the left base. A chest computed tomography scan showed no focal consolidations, pleural effusions, pneumothorax, or pericardial effusions.
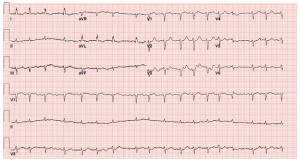
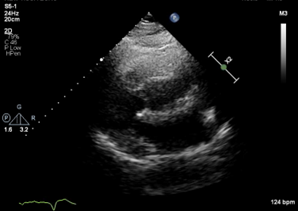
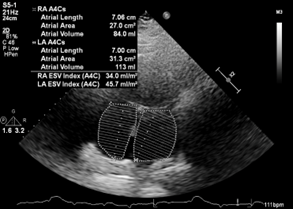
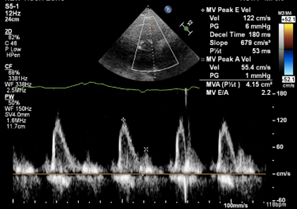
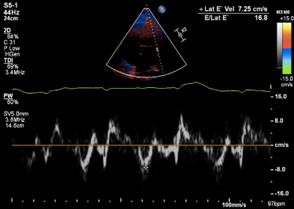
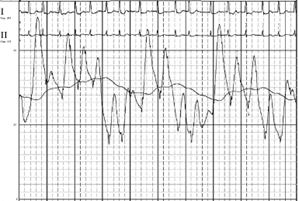
His electrocardiogram showed atrial fibrillation, low voltage QRS segments, and T wave inversions in the lateral limb leads (Figure 1). Transthoracic echocardiogram showed a preserved left ventricular (LV) ejection fraction (EF) of 65%, normal LV size and global wall motion, severely dilated left atrium (LA), dilated right atrium (RA), dilated and hypokinetic right ventricle (RV), mild tricuspid regurgitation, elevated pulmonary artery systolic pressure, and severe diastolic dysfunction (Figure 2). The interventricular septal thickness was 1.1 cm, mitral valve had trace regurgitation, and aortic valve was normal in size without any regurgitation or stenosis. He had a nuclear medicine PYP scan that was negative for TTR amyloidosis.
He obtained a cardiac magnetic resonance imaging scan that showed no regions of abnormal late gadolinium enhancement. He received a nuclear medicine stress test that showed no evidence of myocardial ischemia and a nuclear medicine lung scan that showed no evidence of pulmonary embolism.
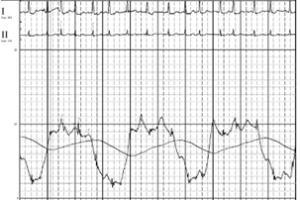
Finally, he obtained a right heart catheterization (Figure 3) that showed elevated filling pressures (mean RA pressure of 14 mmHg, mean pulmonary artery pressure of 39 mmHg, pulmonary capillary wedge pressure of 22 mmHg, LV end diastolic pressure of 18 mmHg, pulmonary vascular resistance of 2 Wood units, and cardiac output of 6.1 L/min/m2).
HFpEF exacerbation was diagnosed as the explanation of his fatigue and dyspnea on exertion. Due to exclusion of other causes such as amyloidosis, hypertrophic cardiomyopathy, restrictive cardiomyopathy, and valvular heart disease from numerous diagnostic studies, obesity was thought to be the major contributing factor to his disease. [1].
Figure 1. Electrocardiogram on admission showing atrial fibrillation, low voltage, and T-wave inversions in lateral leads.
Figure 2. (A) Parasternal long axis view with poor acoustic windows. (B) Apical 4-chamber view demonstrating elevated left and right atrial volumes, 45.7 ml/m2 and 34 ml/m2. (C) Restrictive mitral blood flow velocity (E/A ratio of 2.2) and (D) stiffened mitral annular motion (E/e’ of 16.8) demonstrating severe diastolic dysfunction.
Figure 3. (A) Elevated pulmonary artery pressures (Systolic 48 mmHg, D iastolic 30 mmHg, Mean 39 mm Hg). (B) Elevated pulmonary capillary wedge pressures (22 mmHg).
Epidemiology and Clinical significance
Obesity is a worldwide epidemic. The prevalence of obesity in the United States for example was 15% in 1970, but has now skyrocketed to over 35%.[2] By 2030, the prevalence of obesity is projected to be 50% of Americans.[2]
It was previously taught that the predominant form of HFpEF was from aging and hypertension, which led to stiffened muscles, restrictive vasculature, and diastolic dysfunction.[3] However, patients with obesity-related HFpEF are increasing in prevalence,underdiagnosed, younger in age, have worse quality of life, have more comorbidities, and have worse outcomes compared to those with other forms of HFpEF.[4-5]
It was also previously thought that obese patients with signs and symptoms of HFpEF are merely deconditioned[6]. There have been several barriers to embracing obesity-related HFpEF as a distinct etiology. Patients with significant obesity have been commonly excluded from large heart failure clinical trials and are commonly stigmatized in clinical practice[6]. Additionally, the distinct mechanisms linking link excess adipose tissue causing heart failure has only been recently elucidated[7].
Pathophysiology of Obesity-related HFpEF
The distinct pathophysiology of how obesity causes HFpEF is complex and multifactorial. Obesity increases total blood volume expansion,[8] accelerates cardiac aging by increasing diastolic stiffness,[9] increases triglyceride accumulation in cardiac muscle causing organ dysfunction (similar to how triglyceride accumulation in liver causes hepatic steatosis),[10] decreases myocardial energy efficiency as cardiac tissue consumes more fat compared to glucose for energy,[11] increases coronary microvascular dysfunction by decreasing coronary flow reserve,[12] and increases pericardial fat which increases external pressure on the heart.[13]
Consequently, patients with obesity-related HFpEF have greater difficulty accommodating to stressors such as exercise, fluid overload, and atrial fibrillation. Exercise increases myocardial wall stress by increasing sympathetic tone and increasing afterload. Since patients with obesity-related HFpEF have poor coronary reserve and increased diastolic dysfunction, exercise leads to increased left atrial and pulmonary venous pressures causing further dyspnea.[14-15] Similarly, atrial fibrillation compounds this effect by creating poor atrial contraction, reduced ventricular filling, and greater left atrial and pulmonary venous pressures, thus exacerbating dyspnea.[15-16] Patients tend to respond to these stressors by becoming more sedentary which helps improve dyspnea symptoms as increased venous compliance and lymphatic drainage reduce the fluid in alveoli, but overall worsens the adverse cardio-metabolic dysfunction caused by obesity, as previously described.[17]
Diagnostic Challenges
The diagnosis of an obesity-related HFpEF exacerbation in this patient was clinically challenging and was only obtained after many imaging studies ruled out other etiologies of dyspnea and invasive hemodynamic measurements demonstrated elevated filling pressures.
Although the diagnosis of heart failure is primarily a clinical diagnosis reliant on history and physical examination, many patients do not exhibit the complete set of signs and symptoms for heart failure exacerbation, as was the case in our patient. In fact, fewer than 50% of patients with HFpEF exhibit edema, JVD, crackles, PND, or dyspnea at rest.[18] These latter symptoms are sometimes absent because pulmonary venous capacitance and lymphatic drainage increase over time in response to chronic volume overload.[19] Furthermore, even though the symptoms of fatigue and dyspnea on exertion are sensitive in HFpEF, these symptoms are not specific and can be the result of other many illnesses such as angina, valvular disorders, and obstructive lung diseases.[20]
Weight monitoring is challenging in patients with obesity-related HFpEF. Patients with obesity-related HFpEF have greater daily fluctuations in their body weight[21] and it is difficult to ascertain whether weight gain is from increased adipose tissue or volume overload. B-natriuretic peptide levels are often measured in patients to help diagnose heart failure exacerbations, with BNP levels < 300 pg/ml having a 99% negative predictive value for ruling out acute decompensated HfrEF [22]. However, patients with obesity have smaller left ventricular diameters and thicker walls, thus lower intraventricular pressures, lower wall stress, and thus lower stimuli for BNP production.[23] Obesity also leads to increased production of pro-inflammatory cytokines, neprilysin, and circulating leptin, which all act to degrade BNP levels.[24] Patients with obesity-related HFpEF and normal BNP levels have worse prognosis than all other heart failure patients with similar BNP levels[25]. Imaging modalities such as transthoracic echocardiography (TTE) are often limited in obese patients as the heart is at a deeper depth from the surface and there is greater epicardial fat, which limit imaging resolution.[26] Therein lies the issue of research and clinical care of patients with HFpEF: there is no single, non-invasive predictor of ensuring an accurate diagnosis, as it is a diagnosis of exclusion.
Recently, Reddy et al. published the H2FPEF score to help diagnose HFpEF as the cause of unexplained dyspnea in patients with obesity as opposed to treating this condition as a diagnosis of exclusion[27]. This score is the sum of points based on the following clinical characteristics: Heavy (BMI > 30 kg/m2, 2 points), Hypertensive (hypertension and treated with at least two antihypertensive medications, 1 point), atrial Fibrillation (3 points), Pulmonary hypertension (pulmonary artery systolic pressure > 35 mmHg, 1 point), Elder (Age > 60, 1 point), Filling pressure (E/e’ > 9, 1 point).[27] This score was tested and validated in patients with HFpEF at the Mayo Clinic and in independent cohorts, with invasive hemodynamic measurements from right heart catheterization as the gold standard.[27-28] Patients with dyspnea and a H2FPEF score above 6/9 had a greater than 90% probability of obesity-related HFpEF exacerbation.[27-28] Our patient, for reference, had a H2FPEF score of 9/9, and thus had a greater than 99% probability of HFpEF exacerbation.
Treatment
Compared to HFrEF, medical management of patients with HFpEF is more clinically challenging with fewer options that improve mortality[29]. There have been recent trials that showed sodium/glucose cotransporter-2 inhibitors, mineralocorticoid receptor antagonists, and angiotensin receptor-neprilysin inhibitors can reduce heart failure exacerbations in patients with HFpEF, but it is unclear to what extent these therapies help in patients with obesity-related HFpEF[29]. Current guidelines recommend reduction of underlying risk factors and chronic diseases such as hypertension, atrial fibrillation, and diabetes. Recent studies suggest that aggressive weight reduction through diet, exercise, and bariatric surgery can reduce LV mass, reduce inflammation, improve exercise capacity, improve oxygen consumption, and improve quality of life in patients[30-31]. There are also ongoing trials to investigate the effectiveness of other medications like glucagon-like peptide-1 analogues[32] and soluble guanylate cyclase stimulators[33] for improving symptoms in patients with obesity-related HFpEF.
Take home points
- Obesity-related HFpEF is underdiagnosed and increasing in prevalence, particularly in younger populations
- Obesity causes HFpEF through multiple etiologies including increased myocardial stiffening, volume overload and maldistribution, chronic inflammation, and metabolic demand.
- Diagnosis of obesity-related HFpEF is challenging due to poor sensitivity and specificity of clinical signs and symptoms and technical limitations of cardiovascular imaging
- The H2FPEF score can aid in diagnosing HFpEF as a cause of unexplained dyspnea in patients with obesity
- Treatment of obesity-related HFpEF is challenging; many patients can improve their cardiac function and symptoms through aggressive weight loss.
Sohail Zahid MD, Ph.D is an internal medicine resident at NYU Langone Health and Waqas Haque MD is an internal medicine resident at NYU Langone Health
Peer Review by Bernard Kadosh, MD,Assistant Professor, Department of Medicine at NYU Grossman School of Medicine, Associate Director, Fellowship Training Program for Advanced Heart Failure and Transplant Cardiology
Image courtesy of Wikimedia Commons, source: A humorous comparison between the obese Daniel Lambert and C Wellcome L0020234.jpg
References
- Bozkurt B, Coats AJ, Tsutsui H, et al. Universal Definition and Classification of Heart Failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail. 2021.
- Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N Engl J Med. 2019;381(25):2440-50.
- Kitzman DW. Diastolic Heart Failure in the Elderly. Heart Failure Reviews. 2002;7(1):17-27.
- Reddy YNV, Rikhi A, Obokata M, et al. Quality of life in heart failure with preserved ejection fraction: importance of obesity, functional capacity, and physical inactivity. European Journal of Heart Failure. 2020;22(6):1009-18.
- Reddy YNV, Borlaug BA. Readmissions in Heart Failure: It’s More Than Just the Medicine. Mayo Clinic Proceedings. 2019;94(10):1919-21.
- Kitzman DW, Lam CSP. Obese Heart Failure With Preserved Ejection Fraction Phenotype: From Pariah to Central Player. Circulation. 2017;136(1):20-3.
- Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure With Preserved Ejection Fraction. Circulation. 2017;136(1):6-19.
- Feldschuh J, Enson Y. Prediction of the normal blood volume. Relation of blood volume to body habitus. Circulation. 1977;56(4 Pt 1):605-12.
- Wohlfahrt P, Redfield MM, Lopez-Jimenez F, et al. Impact of general and central adiposity on ventricular-arterial aging in women and men. JACC Heart Fail. 2014;2(5):489-99.
- Unger RH, Orci L. Diseases of liporegulation: new perspective on obesity and related disorders. FASEB J. 2001;15(2):312-21.
- Peterson LR, Herrero P, Schechtman KB, et al. Effect of Obesity and Insulin Resistance on Myocardial Substrate Metabolism and Efficiency in Young Women. Circulation. 2004;109(18):2191-6.
- Bajaj NS, Osborne MT, Gupta A, et al. Coronary Microvascular Dysfunction and Cardiovascular Risk in Obese Patients. J Am Coll Cardiol. 2018;72(7):707-17.
- Verbrugge FH, Guazzi M, Testani JM, Borlaug BA. Altered Hemodynamics and End-Organ Damage in Heart Failure: Impact on the Lung and Kidney. Circulation. 2020;142(10):998-1012.
- Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3(5):588-95.
- Obokata M, Olson TP, Reddy YNV, et al. Haemodynamics, dyspnoea, and pulmonary reserve in heart failure with preserved ejection fraction. Eur Heart J. 2018;39(30):2810-21.
- Zakeri R, Borlaug BA, McNulty SE, et al. Impact of atrial fibrillation on exercise capacity in heart failure with preserved ejection fraction: a RELAX trial ancillary study. Circ Heart Fail. 2014;7(1):123-30.
- Hegde SM, Claggett B, Shah AM, et al. Physical Activity and Prognosis in the TOPCAT Trial (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist). Circulation. 2017;136(11):982-92.
- Solomon SD, Rizkala AR, Lefkowitz MP, et al. Baseline Characteristics of Patients With Heart Failure and Preserved Ejection Fraction in the PARAGON-HF Trial. Circ Heart Fail. 2018;11(7):e004962.
- Reddy YNV, Obokata M, Wiley B, et al. The haemodynamic basis of lung congestion during exercise in heart failure with preserved ejection fraction. Eur Heart J. 2019;40(45):3721-30.
- Mant J, Doust J, Roalfe A, et al. Systematic review and individual patient data meta-analysis of diagnosis of heart failure, with modelling of implications of different diagnostic strategies in primary care. Health Technol Assess. 2009;13(32):1-207, iii.
- Li Y, Yu Y, Wu Y, et al. Association of Body-Weight Fluctuation With Outcomes in Heart Failure With Preserved Ejection Fraction. Front Cardiovasc Med. 2021;8:689591.
- Januzzi JL, Jr., Camargo CA, Anwaruddin S, et al. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol. 2005;95(8):948-54.
- Iwanaga Y, Nishi I, Furuichi S, et al. B-Type Natriuretic Peptide Strongly Reflects Diastolic Wall Stress in Patients With Chronic Heart Failure: Comparison Between Systolic and Diastolic Heart Failure. Journal of the American College of Cardiology. 2006;47(4):742-8.
- Reinmann M, Meyer P. B-type natriuretic peptide and obesity in heart failure: a mysterious but important association in clinical practice. Cardiovascular Medicine. 2020;23(01).
- Verbrugge FH, Omote K, Reddy YN, et al. Heart failure with preserved ejection fraction in patients with normal natriuretic peptide levels is associated with increased morbidity and mortality. European Heart Journal. 2022.
- Crea P, Zito C, Cusma Piccione M, et al. The role of echocardiography in the evaluation of cardiac damage in hypertensive obese patient. High Blood Press Cardiovasc Prev. 2015;22(1):23-7.
- Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A Simple, Evidence-Based Approach to Help Guide Diagnosis of Heart Failure With Preserved Ejection Fraction. Circulation. 2018;138(9):861-70.
- Segar MW, Patel KV, Berry JD, Grodin JL, Pandey A. Generalizability and Implications of the H2FPEF Score in a Cohort of Patients With Heart Failure With Preserved Ejection Fraction. Circulation. 2019;139(15):1851-3.
- Chrysant SG, Chrysant GS. Obesity-related heart failure with preserved ejection fraction: new treatment strategies. Hosp Pract (1995). 2019;47(2):67-72.
- Pandey A, Patel KV, Vaduganathan M, et al. Physical Activity, Fitness, and Obesity in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2018;6(12):975-82.
- Kitzman DW, Brubaker P, Morgan T, et al. Effect of Caloric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA. 2016;315(1):36-46.
- Davies M, Faerch L, Jeppesen OK, et al. Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397(10278):971-84.
- Blanton RM, Takimoto E, Lane AM, et al. Protein kinase g ialpha inhibits pressure overload-induced cardiac remodeling and is required for the cardioprotective effect of sildenafil in vivo. J Am Heart Assoc. 2012;1(5):e003731.