Peer Reviewed
This week’s Primecuts details four examples of novel methodologies for treating common diseases. We begin first with a study repurposing an anti-inflammatory therapeutic for the treatment of atherosclerosis, and move onto a new targeted gene therapy for malignant mesothelioma. Finally, we touch on using probiotics to modify peanut allergies, based on a novel understanding of immunogenicity, and using the immune system to assist regulation in type 1-diabetes. Together, these studies show the power of creative thinking in the scientific process, and how even well characterized pathologies can benefit from new approaches.
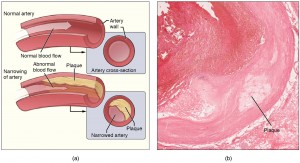
1) Anti-inflammatory Therapy with Canakinumab for Atherosclerotic Disease
Atherosclerosis is well documented to be, at least in part, an inflammatory process (1). The inflammatory response is a critical part of healing after vascular injury, and in the long run likely plays a role in thrombosis and plaque instability (2). However, no purely anti-inflammatory drugs are used for treatment of atherosclerotic disease, and even drugs with anti-inflammatory effects, such as aspirin, are thought to exert their benefit via non-inflammatory mechanisms.
In a recent paper (CANTOS), Ridker and colleagues explore the potential for canakinumab, a monocolonal antibody targeting interleukin-1B, in the treatment of atherosclerotic cardiovascular disease (3). The authors randomly assigned 10,061 patients with previous myocardial infarction and elevated C-reactive protein levels to either one of three doses of canakinumab (50mg, 150mg, 300mg) or placebo. The primary endpoint of this 5 year study was a composite of nonfatal myocardial infarction, nonfatal stroke, and cardiovascular death. The incidence rate for the primary endpoint was 4.50 events per 100 person-years in the placebo group, 4.11 (HR 0.93 [0.80-1.07], p=0.30) in the 50mg group, 3.86 (HR 0.85 [0.74-0.98] p=0.021) in the 150mg group, and 3.90 (HR 0.86 [0.75-0.99] p=0.031) events in the 300mg group. Significantly more infection related deaths occurred in the experimental group.
CANTOS has helped elucidate the inflammatory hypothesis of atherosclerotic cardiovascular disease. However, more studies on the nature of the benefit are needed: statistical significance on the primary composite endpoint was driven by a lower incidence of myocardial infarction alone. And, given that the drug currently costs about $200,000 a year, more efforts are needed to stratify patients for those who may be better responders especially with such a small absolute risk reduction. Nevertheless, with intriguing CANTOS results, the door is open for further studies on anti-inflammatory agents for treatment of atherosclerosis.
2) Long-term clinical and immunological effects of probiotic and peanut oral immunotherapy after treatment cessation: 4-year follow-up of a randomized double-blind placebo-controlled trial
Food allergies have become increasingly common in the past few decades, with deleterious effects on health and quality of life (4). Research shows that children with allergies have a quality of life worse than those with diabetes, and that accidental ingestion of peanuts in those with allergy is the leading cause of anaphylaxis (5, 6).
This week, Tang et al. published 4 year follow-up results from their initial study using a combined probiotic and peanut oral immunotherapy (PPOIT) to desensitize children with peanut allergy. The original trial randomized children into two groups: one with PPOIT with increasing amounts of peanut protein, and the other to placebo. The trial ran for 18 months and found that 82 percent of children in the experimental group became desensitized to peanut, as compared to just 4 percent in the placebo group (tolerance defined by mg of peanut ingestion).
Children from the original study were invited to participate in the extension study, which assessed peanut consumption habits over the last 4 years. The follow-up showed that participants from the PPOIT group were significantly more likely than those from the placebo group to continue eating peanut (16 of 24 (67%) patients versus one of 24 (4%), p=0.001), and had smaller wheals in peanut skin prick tests (5.2m [10.3, 0.0]).
Despite not having any particular guidelines during the follow-up period, treated children were still able to maintain their tolerant state. The trial suggests that continuous, small interventions, can have significant effects for this widely prevalent pathology.
3) Rivaroxaban with or without aspirin in stable cardiovascular disease
Aspirin and if necessary, dual anti-platelet therapy (DAPT) are currently standard of care for secondary prevention in cardiovascular disease. Yet with this regimen, nearly 10% of patients each year have recurrent events (7). In light of this, studies have attempted other means of anti-coagulation, but were met with limited success. Treatment with warfarin, for example, alone or in combination with aspirin, is superior to aspirin alone but leads to a significantly higher rate of intracranial bleeding (8).
Yusuf and colleagues this week reported the results of the Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS) trial, which used the factor Xa inhibitor rivaroxban in secondary prevention (9). This trial randomized 27,395 patients into either low dose aspirin monotherapy (control group), low dose (5mg twice daily) rivaroxaban alone, or aspirin plus very low-dose rivaraoxaban (2.5mg twice daily). The primary endpoint was the rate of a composite endpoint of cardiovascular death, myocardial infarction, or stroke.
The results were compelling. The primary outcome occurred in 4.1% of patients with combination therapy, 4.9% of patients with rivaroxban alone, and 5.4% of patients with aspirin alone (HR of combination therapy vs aspirin alone was 0.76 [0.66, 0.86], p<0.001). Though the rates of major bleeding were significantly higher in the rivaroxaban groups than in the aspirin alone group, there was a net clinical benefit of adding rivaroxaban, and the trial was stopped early for efficacy.
Though the results are compelling, it is noteworthy that the inclusion criteria did not specify that patients be on maximal medical therapy prior to enrollment. This risks the notion that these patients may have been predisposed to a higher rate of events, in comparison to those patients on standard of care for cardiovascular disease. Nevertheless, the trial represents an important step in the field of thrombo-cardiology.
4) Metabolic and immune effects of immunotherapy with proinsulin peptide in human new-onset type 1 diabetesÂ
Type 1 diabetes, which affects over 1 million people in the US, is an autoimmune disorder in which the body’s T-cells mistakenly attack the insulin producing cells in the pancreas (10). Despite the knowledge that type 1 diabetes is an immune mediated disease, few studies have tested the potential merit of immunotherapy in this condition.
Ali and colleagues this week reported the results of a placebo controlled study to determine whether a proinsulin peptide could modify T-cells in patients recently diagnosed with type 1 diabetes (11). 24 patients with the HLA-DRB1*0401 subtype of type 1 diabetes were randomized to either intradermal injections of proinsulin peptide every 2 or 4 weeks, or to placebo. The endpoint of the study was the change in required insulin use over 1 year, and in the serum levels of C-peptide. While the placebo group’s daily insulin use increased by 50% over 12 months, the experimental group remained unchanged. Placebo subjects similarly showed a significant decline in C-peptide versus baseline, whereas no change was seen in those patients on immunotherapy. There was no evidence of toxic side effects, and pancreatic beta cells were not impaired as a result of the therapy.
Though the trial was not powered for efficacy, the results are compelling. The clean safety profile of the immunotherapy provides strong rationale for a subsequent study to more robustly test efficacy, and potentially lead to a new mechanism for treatment of this chronic, prevalent disease.
MinicutsÂ
Natriuretic peptide in congestive heart failure
Natriuretic peptides are biochemical markers of heart failure severity and predictors of adverse outcomes. Results published this week in JAMA showed however that a strategy of titrating standard therapy with natriuretic peptide levels did not significantly improve time to first hospitalization or mortality in heart failure patients (12).
A monoclonal antibody for hemophilia A
Emicuzamab is a monoclonal antibody which functions to bring together clotting factors IX and X, to restore to the function of factor VIII. Results of a phase 3 trial using emicuzamab as prophylaxis in hemophilia A were published, which showed significant benefits in annualized bleeding, as compared to patients receiving standard of care (13).
Selective internal radiotherapy in colorectal cancer
Results from a meta-analysis of three randomized trials using first-line chemotherapy with selective internal radiation therapy (SIRT) in patients with metastatic colorectal cancer with liver metastases was published in The Lancet (14). The data showed that the addition of SIRT to first line chemotherapy did not improve overall survival compared to chemotherapy alone.
Dr. Rushad Dordi is a 1st year resident at NYU Langone Health
Peer reviewed by Neil Shapiro, MD, Editor-In-Chief, Clinical Correlations
Image courtesy of Wikimedia Commons
References
- Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340(2):115-26. http://www.nejm.org/doi/full/10.1056/NEJM199901143400207
- Libby P, Ridker PM, Hansson GK, Leducq Transatlantic Network on A. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54(23):2129-38. https://www.ncbi.nlm.nih.gov/pubmed/19942084
- Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017. http://www.nejm.org/doi/pdf/10.1056/NEJMoa1707914
- Osborne NJ, Koplin JJ, Martin PE, Gurrin LC, Lowe AJ, Matheson MC, et al. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J Allergy Clin Immunol. 2011;127(3):668-76 e1-2. https://www.ncbi.nlm.nih.gov/pubmed/21377036
- Prescott SL, Pawankar R, Allen KJ, Campbell DE, Sinn J, Fiocchi A, et al. A global survey of changing patterns of food allergy burden in children. World Allergy Organ J. 2013;6(1):21.
- Sicherer SH, Sampson HA. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133(2):291-307; quiz 8.
- Bhatt DL, Eagle KA, Ohman EM, Hirsch AT, Goto S, Mahoney EM, et al. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304(12):1350-7.  https://www.ncbi.nlm.nih.gov/pubmed/20805624
- Anand SS, Yusuf S. Oral anticoagulants in patients with coronary artery disease. J Am Coll Cardiol. 2003;41(4 Suppl S):62S-9S.
- Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, et al. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N Engl J Med. 2017.
- Patterson CC, Gyurus E, Rosenbauer J, Cinek O, Neu A, Schober E, et al. Trends in childhood type 1 diabetes incidence in Europe during 1989-2008: evidence of non-uniformity over time in rates of increase. Diabetologia. 2012;55(8):2142-7. https://www.ncbi.nlm.nih.gov/pubmed/22638547
- Alhadj Ali M, Liu YF, Arif S, Tatovic D, Shariff H, Gibson VB, et al. Metabolic and immune effects of immunotherapy with proinsulin peptide in human new-onset type 1 diabetes. Sci Transl Med. 2017;9(402). https://www.ncbi.nlm.nih.gov/pubmed/28794283
- Felker GM, Anstrom KJ, Adams KF, Ezekowitz JA, Fiuzat M, Houston-Miller N, et al. Effect of Natriuretic Peptide-Guided Therapy on Hospitalization or Cardiovascular Mortality in High-Risk Patients With Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA. 2017;318(8):713-20.
- Oldenburg J, Mahlangu JN, Kim B, Schmitt C, Callaghan MU, Young G, et al. Emicizumab Prophylaxis in Hemophilia A with Inhibitors. N Engl J Med. 2017;377(9):809-18. https://www.ncbi.nlm.nih.gov/pubmed/28691557
- Wasan HS, Gibbs P, Sharma NK, Taieb J, Heinemann V, Ricke J, et al. First-line selective internal radiotherapy plus chemotherapy versus chemotherapy alone in patients with liver metastases from colorectal cancer (FOXFIRE, SIRFLOX, and FOXFIRE-Global): a combined analysis of three multicentre, randomised, phase 3 trials. Lancet Oncol. 2017. https://www.ncbi.nlm.nih.gov/pubmed/28781171