Peer Reviewed
In May 2012, The U.S. Department of Health and Human Services unveiled the “National Plan to Address Alzheimer’s Disease,†in response to legislation signed by President Obama in January, 2011 establishing the National Alzheimer’s Project Act. The overarching goal of the Plan is to “prevent or effectively treat Alzheimer’s Disease by 2025.â€Â  We now have an estimated 5.5 million people who are living with this illness[1] and these numbers are only expected to grow with the aging population̶ the prevalence of Alzheimer’s disease (AD) doubles for every 5-year interval over the age of 65[2]. The U.S. Preventive Services Task Force finds no evidence to recommend widespread dementia screening[3], however the Affordable Care Act requires an annual cognitive assessment for all Medicare enrollees. It is troublesome that, in one study 55% of cases of dementia went unrecognized by patients’ primary care physicians.[4] While a definitive diagnosis of AD remains a histopathologic one, clinicians must be able to correctly diagnose AD clinically in order to provide patients with an appropriate diagnosis and crucial supportive care prior to clinical deterioration. Over nine hundred million dollars were allocated by the NIH in 2016[5] to work toward the stated goal of effective treatment or prevention of Alzheimer’s in the next 10 years, so identifying the patients who will benefit from these treatments will become even more important in the years to come. Primary care providers are tasked with recognizing the early signs of cognitive decline in their patients. This review seeks to identify the tools available to primary care providers to aid in-office diagnosis of AD, and to determine how effective these tools are at detecting AD. In addition, this review examines the current role of neuroimaging in the diagnosis of AD, and looks at what advances have been made in imaging that might become more widespread in the future. Finally, the most promising biomarkers for early detection of AD are outlined here, though none are validated for use in clinical practice currently.
In 2011, the Alzheimer’s Association, the National Institute of Aging, and the National Institute of Health convened expert workgroups, which, for the first time since 1984, set out new criteria and guidelines for the diagnosis of Alzheimer’s disease.[6] These new guidelines divide AD into three phases. The first is known as pre-clinical Alzheimer’s disease, where biomarker changes in the brain and CSF may occur years before cognitive symptoms appear. This phase is currently not relevant to clinical practice, but rather anticipates the research and development of accurate biomarkers that could eventually diagnose AD in its early stages, the time at which novel treatments may be most effective.[7] The second phase of Alzheimer’s disease, mild cognitive impairment (MCI), is present in the patient who has experienced gradually progressive cognitive decline, however has not yet reached a level of impairment such that there is interference of daily function, which is characteristic of dementia.[8] It is important in patients with MCI to evaluate for non-AD etiologies of cognitive decline, including vascular, traumatic, metabolic, iatrogenic or psychiatric. Not all patients with MCI progress to AD[9], and a great deal of research is underway to help determine what factors predispose a person with MCI to progress to dementia.  Finally, Alzheimer’s dementia is the third phase of AD proposed by the 2011 workgroups and is characterized by a decline in cognitive or neuropsychiatric function from previous, such that these symptoms interfere with the ability to function at work or with a patient’s usual activities.
AD is generally insidious in onset over months to years, and most often presents with memory dysfunction (though language, visuospatial, or executive dysfunction can also be the presenting symptom). The workgroup further broke down the classification for diagnoses into probable AD dementia, probable AD dementia with increased level of certainty, and possible AD dementia. Probable AD dementia was defined as cognitive decline that has an insidious onset and a clear-cut history of worsening of cognition by report or observation. The most common initial symptom of cognitive decline is an amnestic presentation including impairment in retention and recall of recently learned information. However, there also must be evidence of dysfunction in at least one other cognitive domain: executive function, visuospatial abilities, language abilities or personality and behavior. Dysfunction in one of these other domains is less commonly the primary presenting symptom. In addition, there should be no evidence of a mixed etiology (ie, cerebrovascular disease). Probable AD dementia with increased level of certainty includes patients with documented decline on subsequent evaluations based on information from informants and standardized cognitive testing. It also includes patients who meet the core clinical criteria for probable AD who have a causative genetic mutation (in APP, PSEN1, PSEN2). The workgroup noted that ApoEε4 allele was not specific enough to be included in this category. A patient with possible AD dementia has cognitive decline that meets criteria for AD, but presents with decline that is either more rapid than unexpected or where decline has not been documented. Likewise, when there is evidence of a possible mixed etiology (concomitant cerebrovascular disease or features of Lewy body dementia), the patient is considered to have possible AD.[10]
In order to accurately diagnose either MCI or AD, practitioners often use freely available and validated in-office assessments to assess cognitive function. The National Institute on Aging has compiled on its website a database of 116 instruments to detect cognitive impairment in older adults in a variety of languages, times for administration, and some have been evaluated in different populations and races.[11] But which of these tools is best for the primary care setting?
Cochrane has conducted a series of systematic reviews to evaluate the evidence behind several popular neuropsychololgical tests for the detection of Alzheimer’s disease. One such study found that the Mini-Mental Status Examination (MMSE), using a conventional cutoff score of 24, had a sensitivity of 0.85 (95% CI:0.74-0.92) and specificity of 0.90 (95% CI:0.82-0.95) in detecting dementia. Results for a cut point of 25 were similar.[12] This study acknowledges the limitation that the MMSE is used less and less in clinical practice due to increased enforcement of that tool’s copyright. Another review in the Cochrane series was somewhat limited in that a meta-analysis was not performed due to inter-study variation. This review found that the popular Montreal Cognitive Assessment (MoCA) was very sensitive (0.94 and above), but had low specificity (0.60 and below) at the traditional cut point of 25/26, and thus was not recommended for clinical practice[13]; however one study within that review found more balanced sensitivities and specificities when cut points were adjusted for education. A study in 2006 found that the Mini-Cog (a 3-item recall task and an elective clock-drawing task) was more sensitive than physician recognition at detecting cognitive impairment at all levels of impairment except for severe dementia. In subjects with probable AD dementia, the Mini-Cog detected 99% of cases as compared with 62% detected by physicians (P<0.01).[14]
Helpfully, in 2013 the Alzheimer’s Association (AA) published an algorithm to assist in evaluation for cognitive impairment within the scope of the Medicare Annual Wellness Visit, with some decision points based on whether an informant (family member, caregiver, etc.) was available. [15] The AA selected the Mini-Cog, the General Practitioner Assessment of Cognition (GPCOG) (sensitivity 0.82, specificity 0.83)[16]  and the Memory Impairment Screen (MIS) (at a cut point of 4, sensitivity was 0.80 and specificity 0.96) [17] as the best-suited tests for routine use in primary care. Their decision factored in several characteristics such as validation in a primary care setting and administration time (5 minutes or less). They also note that the GPCOG, Short IQCODE[18] and the AD8[19] are the most well-validated tools to use with informant-reported data. Scores above or below certain set points on these assessments should trigger a full dementia evaluation or a referral for such evaluation.
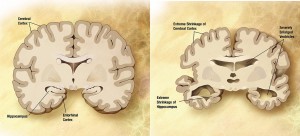
The use of neuroimaging in the workup of patients with probable or possible AD has generally been as a tool to exclude other intracranial pathologies that could mimic the symptoms of AD.  The American College of Radiology Appropriateness Criteria recommends MRI of the head without contrast in initial evaluation.[20] This modality is also supported by the European Federation of the Neurological Societies, as it allows for ruling-out of structural abnormalities such as tumors and subdural hematomas, and evaluation of any cerebrovascular disease that may contribute to symptoms.  It can also demonstrate patterns of cerebral atrophy consistent with certain types of dementia – for AD, involvement of medial temporal lobes, especially hippocampal atrophy, is most prominent[21].  A baseline MRI to evaluate structural changes was slightly better at predicting cognitive decline than certain well-validated CSF biomarkers (t-tau/ Aβ42)[22]. This increased accuracy and less invasive nature make MRI a useful tool for early detection of AD. The nuclear medicine tool single photon emission CT (SPECT) can evaluate areas of hypoperfusion that, in one study, were larger than corresponding areas of atrophy (that correlated with subjects’ known clinical deficits) on MRI.[23]. This suggests a synergistic benefit to imaging with these two techniques. Combining multiple modalities of neuroimaging could identify AD cases early in the disease process, and better differentiate other types of dementia, such as vascular and frontotemporal dementia, in instances where the etiology is not entirely clear. [24] The use of [18F] fludeoxyglucose(FDG)-PET to show areas of hypometabolism can be useful in predicting progression from MCI to AD, and is available to Medicare recipients to assist in the diagnosis of dementia; however, it is not recommended for routine diagnosis of AD[20]. Hypometabolism in certain regions of the brain as determined by FDG-PET was more pronounced in MCI patients who went on the convert to AD than it was for MCI patients who did not develop AD. Likewise, PET detection of Aβ amyloid deposition by florbetapir tracer PET has been used in research, however one study showed that up to 14% of cognitively normal subjects were amyloid positive using this method.[25]
In the quest for the accurate and early diagnosis of AD, researchers have examined multitudes of potential serum, plasma and CSF biomarkers. Recently, the Lancet Neurology published a meta-analysis looking at 15 of the most commonly studied CSF and blood biomarkers for AD.  The neuropathologic diagnosis of AD in autopsy shows both amyloid plaques and neurofibrillary tangles, the latter made up of abnormal aggregations of tau proteins.[26] The Lancet Neurology review showed strong evidence that AD is associated with lower CSF levels of Aβ42 and higher CSF levels of T-tau and P-tau proteins when compared with controls. The drop in Aβ42 CSF levels may precede the diagnosis of AD dementia by 10 years[27]. Another promising CSF biomarker is an increased level of CSF NFL (neurofilament light protein) which represents axonal degeneration. In a Lancet meta-analysis, this biomarker was shown to have an average ratio of 2.35 (95% CI:1.90-2.91) in subjects with AD versus controls. Plasma T-tau levels were significantly increased in AD, however there was considerable inter-study variability on this measure, and the review concluded that more research was needed before recommending this plasma biomarker. Notably, plasma Aβ levels did not correlate significantly with AD.[27]
For the clinician approaching a patient with possible cognitive impairment, the Mini-Cog, MIS and GPCOG, have been shown to be both effective and time-efficient in detecting patients with probable AD. Currently, MRI is recommended chiefly to rule out alternative causes of dementia, but it is helpful in recognizing patterns of atrophy that are suggestive of AD. Further neuroimaging, including PET scans with various radiotracers can help better differentiate AD from other dementias and detect the disease earlier than MRI alone. The importance of early detection is likely to become more important as research aimed at treatments progresses. To this end, biomarkers, including CSF NFL, Aβ42, T-tau and P-tau are currently being researched to determine their efficacy in early diagnosis of disease, as well as identifying people at risk. Ultimately the utility of these biomarkers holds promise in expanding understanding of the pathophysiology of Alzheimer’s disease. All of the modalities examined here for diagnosis can also help to lead to better understanding of the entire disease process, which is a key step in bringing us closer to developing effective treatments for the disease.
Because there is no current medication that is able to treat or modify the course of AD, a great deal of research has given way to neuroimaging in multiple modalities and promising CSF and blood biomarkers with the hope that these tools, separately or more likely in concert with one another, can lead to much earlier diagnoses of AD. Having this early diagnostic capability opens up the possibility for future therapeutic agents that might be able to halt or slow this terrible disease process.
Dr.  Karen McCloskey is a 3rd-year resident at NYU Langone Health
Peer reviewed by Thomas Wisniewski, MD, Neurologist, NYU Langone Health
Image courtesy of Wikimedia Commons
References:
[1]      A. Association, “2017 Alzheimer’s disease facts and figures,†Alzheimer’s Dement, pp. 13:325–373, 2017.
[2]      National Institute on Aging, National Institutes of Health, and U.S. Department of Health and Human Services, “2014-2015 Alzheimer’s Disease Progress Report.â€Â  http://www.questri.com/wp-content/uploads/2016/09/2014-2015_alzheimers-disease-progress-report.pdf
[3]      V. A. Moyer, “Screening for cognitive impairment in older adults: U.S. Preventive Services Task Force recommendation statement.,†Ann. Intern. Med., vol. 160, no. 11, pp. 791–797, Jun. 2014.
[4]      V. Kotagal, K. M. Langa, B. L. Plassman, G. G. Fisher, B. J. Giordani, R. B. Wallace, J. R. Burke, D. C. Steffens, M. Kabeto, R. L. Albin, and N. L. Foster, “Factors associated with cognitive evaluations in the United States.,†Neurology, vol. 84, no. 1, pp. 64–71, Jan. 2015.
[5]      National Institutes of Health, “Estimates of Funding for Various Research, Condition and Disease Categories.†[Online]. Available: https://report.nih.gov/categorical_spending.aspx. [Accessed: 08-Aug-2016].
[6]      C. R. Jack Jr., M. S. Albert, D. S. Knopman, G. M. McKhann, R. A. Sperling, M. C. Carrillo, B. Thies, and C. H. Phelps, “Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease,†Alzheimer’s Dement. J. Alzheimer’s Assoc., vol. 7, no. 3, pp. 257–262, Aug. 2016.
[7]      R. A. Sperling, P. S. Aisen, and L. A. Beckett, “Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease,†Alzheimers Dement, vol. 7, 2011.
[8]      M. S. Albert, S. T. DeKosky, and D. Dickson, “The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging—Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease,†Alzheimers Dement, vol. 7, 2011.
[9]      M. Ganguli, B. E. Snitz, J. A. Saxton, C.-C. H. Chang, C.-W. Lee, J. Vander Bilt, T. F. Hughes, D. A. Loewenstein, F. W. Unverzagt, and R. C. Petersen, “Outcomes of mild cognitive impairment by definition: a population study.,†Arch. Neurol., vol. 68, no. 6, pp. 761–7, 2011. https://www.ncbi.nlm.nih.gov/pubmed/21670400
[10]   G. M. McKhann, D. S. Knopman, and H. Chertkow, “The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease,†Alzheimers Dement, vol. 7, 2011.
[11]   National Institute on Aging, “Instruments to Detect Cognitive Impairment in Older Adults.†[Online]. Available: https://www.nia.nih.gov/research/cognitive-instrumen.
[12]   S. T. Creavin, S. Wisniewski, A. H. Noel-Storr, C. M. Trevelyan, T. Hampton, D. Rayment, V. M. Thom, K. J. E. Nash, H. Elhamoui, R. Milligan, A. S. Patel, D. V Tsivos, T. Wing, E. Phillips, S. M. Kellman, H. L. Shackleton, G. F. Singleton, B. E. Neale, M. E. Watton, and S. Cullum, “Mini-Mental State Examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations.,†Cochrane database Syst. Rev., no. 1, p. CD011145, 2016.
[13]   D. H. J. Davis, S. T. Creavin, J. L. Y. Yip, A. H. Noel-Storr, C. Brayne, and S. Cullum, “Montreal Cognitive Assessment for the diagnosis of Alzheimer’s disease and other dementias.,†Cochrane database Syst. Rev., no. 10, p. CD010775, 2015.
[14]   S. Borson, J. M. Scanlan, J. Watanabe, S.-P. Tu, and M. Lessig, “Improving identification of cognitive impairment in primary care.,†Int. J. Geriatr. Psychiatry, vol. 21, no. 4, pp. 349–355, Apr. 2006.  https://www.ncbi.nlm.nih.gov/pubmed/16534774
[15]   C. B. Cordell, S. Borson, M. Boustani, J. Chodosh, D. Reuben, J. Verghese, W. Thies, and L. B. Fried, “Alzheimer’s Association recommendations for operationalizing the detection of cognitive impairment during the Medicare Annual Wellness Visit in a primary care setting,†Alzheimer’s Dement. J. Alzheimer’s Assoc., vol. 9, no. 2, pp. 141–150, Aug. 2016.
[16]   H. Brodaty, D. Pond, N. M. Kemp, G. Luscombe, L. Harding, K. Berman, and F. A. Huppert, “The GPCOG: a new screening test for dementia designed for general practice.,†J. Am. Geriatr. Soc., vol. 50, no. 3, pp. 530–534, Mar. 2002.
[17]   H. Buschke, G. Kuslansky, M. Katz, W. F. Stewart, M. J. Sliwinski, H. M. Eckholdt, and R. B. Lipton, “Screening for dementia with the memory impairment screen.,†Neurology, vol. 52, no. 2, pp. 231–238, Jan. 1999.
[18]   A. F. Jorm, “A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation.,†Psychol. Med., vol. 24, no. 1, pp. 145–153, Feb. 1994.
[19]   J. E. Galvin, C. M. Roe, C. Xiong, and J. C. Morris, “Validity and reliability of the AD8 informant interview in dementia.,†Neurology, vol. 67, no. 11, pp. 1942–1948, Dec. 2006.
[20]   F. J. 2nd Wippold, D. C. Brown, D. F. Broderick, J. Burns, A. S. Corey, T. K. Deshmukh, A. C. Douglas, K. Holloway, B. D. Jagadeesan, J. S. Jurgens, T. A. Kennedy, N. D. Patel, J. S. Perlmutter, J. M. Rosenow, K. Slavin, and R. M. Subramaniam, “ACR Appropriateness Criteria Dementia and Movement Disorders.,†J. Am. Coll. Radiol., vol. 12, no. 1, pp. 19–28, Jan. 2015.
[21]   M. Filippi, F. Agosta, F. Barkhof, B. Dubois, N. C. Fox, G. B. Frisoni, C. R. Jack, P. Johannsen, B. L. Miller, P. J. Nestor, P. Scheltens, S. Sorbi, S. Teipel, P. M. Thompson, and L. O. Wahlund, “EFNS task force: The use of neuroimaging in the diagnosis of dementia,†Eur. J. Neurol., vol. 19, no. 12, pp. 1487–1501, 2012.
[22]   P. Vemuri, H. J. Wiste, and S. D. Weigand, “MRI and CSF biomarkers in normal, MCI, and AD subjects: diagnostic discrimination and cognitive correlations,†Neurology, vol. 73, 2009.
[23]   R. J. Caselli, C. R. Jack, and R. C. Petersen, “Asymmetric cortical degenerative syndromes: clinical and radiologic correlations,†Neurology, vol. 42, 1992.
[24]   A. R. Varma, W. Adams, and J. J. Lloyd, “Diagnostic patterns of regional atrophy on MRI and regional cerebral blood flow change on SPECT in young onset patients with Alzheimer’s disease, frontotemporal dementia and vascular dementia,†Acta Neurol Scand, vol. 105, 2002.
[25]   Q. Ruan, G. D’Onofrio, D. Sancarlo, Z. Bao, A. Greco, and Z. Yu, “Potential neuroimaging biomarkers of pathologic brain changes in Mild Cognitive Impairment and Alzheimer’s disease: a systematic review,†BMC Geriatr., vol. 16, no. 1, pp. 1–9, 2016.  https://www.ncbi.nlm.nih.gov/pubmed/27184250
[26]   B. T. Hyman, C. H. Phelps, T. G. Beach, E. H. Bigio, N. J. Cairns, M. C. Carrillo, D. W. Dickson, C. Duyckaerts, M. P. Frosch, E. Masliah, S. S. Mirra, P. T. Nelson, J. A. Schneider, D. R. Thal, B. Thies, J. Q. Trojanowski, H. V Vinters, and T. J. Montine, “National Institute on Aging – Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease,†Alzheimer’s Dement. J. Alzheimer’s Assoc., vol. 8, no. 1, pp. 1–13, Aug. 2016.
[27]   B. Olsson, R. Lautner, U. Andreasson, A. Ohrfelt, E. Portelius, M. Bjerke, M. Holtta, C. Rosen, C. Olsson, G. Strobel, E. Wu, K. Dakin, M. Petzold, K. Blennow, and H. Zetterberg, “CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis.,†Lancet. Neurol., vol. 15, no. 7, pp. 673–684, Jun. 2016.