Faculty Peer Reviewed
Diseases 2.0 – Bringing you the latest updates on disease pathophysiology and treatment
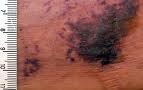
Patient S.J. is a 36 year old female with a 20 year history of lupus and lupus nephritis now with end stage renal disease (ESRD) on hemodialysis. She presented with indurated, violaceous skin lesions with ulceration on both thighs. The lesions worsened and became increasingly painful over the last 6 months. Her extensive four month hospital course has been complicated by numerous episodes of superinfection and sepsis with methicillin resistant Staph aureus treated by intravenous antibiotics and wound debridement and negative pressure wound dressings. Over the last several weeks, her exquisitely painful skin ulcerations continue to worsen and have expanded to involve her trunk.
Calcific uremic arteriolopathy, also called by the older term calciphylaxis, is a rare and devastating disorder which most often occurs in patients with severe renal disease.(1,3) In humans, the term calciphylaxis is used to describe a disorder of the soft tissue characterized by arterial calcification.(1) CUA occurs in up to 5% of patients on hemodialysis.(2) Other risk factors for its development include female gender(2,4), obesity(2,3), diabetes(2), systemic steroid use(3,4), warfarin use (2,3), liver disease(3,4), and calcium-phosphate product of greater than 70 mg2/dL.(2.3,4)Â Â Â Some believe that the disorder is much more frequent in an era in which calcium is used as a first-line phosphate binder, contributing to an increased calcium-phosphate product. The survival rate at one year is just 45.8%.(3)
CUA presents as painful necrotic skin ulcerations. Tissue biopsy, showing characteristic histopathological findings, remains the gold standard for diagnosis.(2) The pathophysiology of this disorder remains unclear, but recent research suggests that multiple factors may ultimately converge to activate the NFκB pathway, leading to bone pathology and vascular calcification.(5) It is thought that the cutaneous necrosis of CUA is ischemic in nature, resulting from thrombotic vascular occlusion of vessels with narrowed lumens secondary to medial arteriolar calcium deposition and subintimal fibrosis with fibrin thrombi. (5)
The best approach to preventing CUA is to employ strategies that limit the development of vascular calcifications in all patients at high risk, which includes ESRD patients. These strategies should include agents that directly or indirectly inhibit the NFκB cascade such as bisphosphonates, recombinant OPG (osteoprotegerin), anti-RANKL antibodies, and BMP.(7, 2, 5) Bisphosphonates are the only option among these that is currently available. The NFκB pathway is integral to the mineral deposition and resorption of bone and plays a role in the regulation of extraosseous mineralization.(3) Bisphosphonates are thought to increase OPG production and thereby inhibit NFκB or RANKL activity,(2) whereas PTH, corticosteroids, aluminum, liver disease, and other forms of inflammation increase RANKL expression, decrease OPG, and activate NFκB.(3)
Once CUA has developed, therapeutic options remain controversial and minimally to moderately effective at best. Few case-control studies exist comparing various treatment options for this disease. One goal of traditional therapy is decreasing the calcium-phosphate product. Agents used for this purpose include phosphate binders like sevelamer, bisphosphonates, and cinacalcet.(1,2) As secondary hyperparathyroidism has been implicated as a contributor to CUA, suppression of PTH has been an unproven goal of therapy. Surgical resection of the parathyroid glands has been used with variable success to improve calcium, phosphate, and parathyroid hormone levels. One small retrospective study in the journal Surgery showed an increase in median overall survival in patients after parathyroidectomy (80 months) compared to patients who had not had a parathyroidectomy (35 months).(6) However, this benefit remains controversial and most authors suggest that parathyroidectomy be reserved for patients with severe hyperparathyroidism that is not responsive to medical correction.(4)  Medical suppression of PTH involves the use of the calcimimetic agent cinacalcet. Although case reports include patients in whom cinacalcet was one of several therapies utilized successfully, its relative importance in therapy is not established. Therapies must also focus on restoring tissue perfusion and improving cutaneous oxygenation.(5) Hyperbaric oxygen, antithrombolytic therapy with tPA, and anticoagulation to maintain vascular patency are used to this effect.(2,4)
Aggressive wound care measures must be implemented. Larval and surgical wound debridement have shown promise in promoting healing and decreasing the incidence of wound infection and sepsis.(4) Larval debridement shows promise as a low-pain, microbe-reducing, adjunctive therapy that spares viable tissue. Surgical debridement appears to improve outcomes with 6.2%Â one-year survival in patients who had had surgical debridement compared to 37.4%Â one- year survival in patients who did not undergo debridement.(3) Wound healing may be promoted through the use of sodium thiosulphate. Sodium thiosulphate is a chelator of cations including calcium and helps to convert calcified deposits into a more soluble form.(1,2) It is also an antioxidant which may help reverse endothelial dysfunction.
Other novel therapies including hematopoietic stem cell transplantation (HSCT) have resulted in resolution of calciphylaxis in case reports. (1)Â However, the fact that such benefit was achieved from HSCT suggests that immunosuppressive therapies may hold promise for targeting the inflammation which appears to be a component of the pathogenesis of this devastating and deadly disease.
Regrettably, despite multiple debridements, broad spectrum antibiotics, anti-thrombolytic therapy, and sevelemar, our patient S.J. eventually succumbed to her CUA, likely secondary to sepsis as a result of wound superinfection. Unfortunately, none of the therapies for CUA offer a definitive cure or are consistently successful. The number of randomized controlled trials looking at outcomes also continues to be very limited. One hopes that some of the recent discoveries related to the pathogenesis of CUA will lead to the development of new strategies to help improve outcomes.
Reviewed by David Goldfarb, MD, Professor of Medicine, NYU Medical Center, Chief Nephrology Section VA New York Harbor
References:
1. Mandelbrot DA, Santos PW, Burt RK et al. Resolution of SLE-related soft-tissue calcification following haematopoietic stem cell transplantation. NDT Advance Access published on August 1, 2008, DOI 10.1093/ndt/gfn036. Nephrol. Dial. Transplant. 23: 2679-2684. http://ndt.oxfordjournals.org/cgi/content/full/23/8/2679
2. Rogers NM. Coates PT. Calcific uraemic arteriolopathy: an update. [Review] [60 refs] [Journal Article. Review] Current Opinion in Nephrology & Hypertension. 17(6):629-34, 2008 Nov.
3. Weenig R, Sewell L, Davis M, et al. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol 2007; 56:569-579.
4. Weenig RH. Gibson LE. el-Azhary R. The role of the hospital dermatologist in the diagnosis and treatment of calciphylaxis and nephrogenic systemic fibrosis. [Review] [20 refs] [Case Reports. Journal Article. Review] Seminars in Cutaneous Medicine & Surgery. 26(3):163-7, 2007 Sep.
5. Weenig RH. Pathogenesis of calciphylaxis: Hans Selye to nuclear factor kappa-B. [Review] [149 refs] [Biography. Historical Article. Journal Article. Review] Journal of the American Academy of Dermatology. 58(3):458-71, 2008 Mar.
6. Arch-Ferrer JE, Beenken SW, Rue LW et al. Therapy for calciphylaxis: An outcome analysis. Surgery – December 2003 (Vol. 134, Issue 6, Pages 941-944.