Faculty peer reviewed
Case:
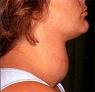
A 44-year old female presented to the emergency room with complaints of a lower extremity rash and swelling. The patient had been in her usual state of health when she presented to her primary care physician with complaints of palpitations, weight loss and insomnia. Lab tests were performed and she was given a diagnosis of hyperthyroidism. She was started on propranolol and methimazole, which the patient took intermittently due to intolerance of side effects, which she perceived as the rash and swelling.Â
INTRO
Graves’ disease is characterized by the presence of hyperthyroidism, goiter, and sometimes ophthalmopathy and dermopathy. It is a condition believed to belong to a spectrum of diseases termed autoimmune thyroid diseases, of which Hashimoto’s thyroiditis, postpartum thyroiditis, silent thyroiditis, and atrophic autoimmune thyroiditis belong. The primary event in autoimmune thyroid diseases is likely T-cell mediated and many of the tissue consequences in both Graves’ and Hashimoto’s disease are caused by specific sub-sets of lymphocytes, lymphokines, as well as antibodies. The T-cell factors induce the B-cells to produce antibodies: Thyroid Stimulating Immunoglobulins in Graves’ and anti-Thyroglobulin, anti-thyroid peroxidase, and anti-thyroid symporter in all autoimmune thyroid disorders, as well as TSH-blocking antibodies. The TSH-blocking antibodies interfere with the ability of native TSH and TSI to up-regulate the TSH receptor. The net thyroid functional effect is the sum of the trophic and inhibitory factors, anti-enzyme effects, and destructive T-Cell phenomenoa .   For example, in Graves’ disease activation of the thyroid stimulating hormone receptor causes increased production of thyroid hormone leading to hyperthyroidism1, and in Hashimoto’s thyroiditis, anti- anti-thyroid peroxidase antibodies block organification of iodine and may lead to reduced T-4/T-3 levels. The major thyroid related antigens that are known to have antibodies made against them are the TSH receptor, thyroglobulin, thyroid peroxidase, and the sodium/iodine symporter.2Â
In pregnant women, an important issue relates to the immunoglobulins crossing the placenta, which could cause transient neonatal (or fetal) hyper- or hypothyroidism.
Here we will discuss the clinical use of thyroid antibody assays in patients with thyroid diseases.
THYROID STIMULATING HORMONE RECEPTOR ANTIBODY
Antibodies to the Thyroid Stimulating Hormone (TSH) receptor have been found in both Graves’ and Hashimoto’s disease patients, however the stimulating variety of antibodies is specific to Graves’ disease. TSH receptor antibody assays have been reported to have a specificity of 99.6% with a sensitivity of 98.8% for Graves’ disease.3  TSH receptor antibodies have been used in various roles such as for diagnosis, as a marker of severity and as an aid for choice of treatment for Graves’ disease, however they are not commonly tested for in North America, mostly due to regional differences in practice and cost-effectiveness.4Â
THYROID STIMULATING ANTIBODY
Thyroid Stimulating antibody assays measure the ability of IgG to bind to TSH receptor on cells and stimulate adenylate cyclase production. They are also referred to as thyroid-stimulating immunoglobulin (TSI) assays. Thyroid stimulating immunoglobulins (TSI) have been detected in 77.8% to 92% of Graves’ patients.5-6  In clinical practice, TSI is not necessary to diagnose Graves’ disease, however it may be used to predict relapse or remission after treatment of Graves’ disease with radioiodine7 or methimazole8, as persistent elevation of TSI correlates with disease activity and remission is normally accompanied by a decrease in activity.
 NOMENCLATURE
Of note, the nomenclature for TSH receptor-antibody assays are known to be inconsistent and confusing.9 Assays that measure binding of TSH to solubilized receptor are referred to as TRAb (thyroid receptor antibody), TBII (TSH-binding inhibitor immunoglobulin), or LATS (long-acting thyroid stimulator). Assays that measure the ability of IgG to bind to TSH receptor on cells and stimulate adenylate cyclase production are referred to as TSI (thyroid-stimulating immunoglobulin) assays. LATS was the first anti-TSH assay, is a heterophile antibody, reacting with mouse, not human thyroid, and is of historical interest only.
ANTI- THYROGLOBULIN and ANTI-THYROID PEROXIDASE ANTIBODIES
Thyroglobulin (Tg) is a large glycoprotein that is synthesized by follicular cells in the thyroid gland and secreted into the lumen of the thyroid follicle. Thyroid peroxidase (TPO) is a key enzyme in the production of thyroid hormone. TPO catalyzes the iodination of tyrosine residues in thyroglobulin to form monoiodotyrosine that goes on to form diiodotyrosine and ultimately thyroxine. Antibodies to Tg (anti-Tg) and TPO (anti-TPO) are present in both Hashimoto’s and Graves’ disease patients.
The American Thyroid Association (ATA) guideline statement on treatment of patients with hypothyroidism recommends testing for either anti-TPO or anti-Tg antibodies when autoimmune thyroiditis is suspected as the underlying cause of hypothyroidism and that the anti-TPO antibody test is the more sensitive and specific of the two tests.10Â
In patients with subclinical hypothyroidism, painless thyroiditis or postpartum thyroiditis, the presence of anti-TPO antibodies may help predict progression to permanent hypothyroidism. In patients with subclinical hypothyroidism and positive anti-thyroid antibodies, the ATA states that treatment of hypothyroidism is probably advisable because of the high likelihood of progression to overt hypothyroidism.10 If treatment is deferred, at least yearly evaluation for clinical or biochemical evidence of hypothyroidism is recommended.
In Hashimoto’s patients, anti-TPO and anti-Tg abs have respective prevalence rates of 90-100% and 80-90%.  In Graves’ patients, anti-TPO and anti-Tg abs have respective prevalence rates of 50-70% and 50-80%.11 These antibodies are not specific to these particular diseases.Â
CONCLUSIONS
In summary, patients with primary hypothyroidism, anti-TPO and anti-Tg antibodies may be obtained to support the diagnosis of autoimmune thyroiditis, with the former test shown to help predict progression of sub-clinical hypothyroidism to overt hypothyroidism. In patients with primary hyperthyroidism, a TSI assay may be obtained to support the diagnosis of Graves’ disease and may be followed as a measure of disease activity. Anti-TPO and anti-Tg antibodies may be present in Graves’ disease patients as an epiphenomenon, but are not causative, in contrast to TSI, which is responsible for the excessive production of T-4/T-3. In addition, the TSI, anti-TPO, and TSH blocking antibodies are helpful in predicting the development of transient neonatal Graves’ disease or hypothyroidism in pregnant women with Graves’ disease or Hashimoto’s thyroiditis.Â
RESOLUTION OF CASE
The patient was thought to have a drug rash induced by methimazole, which was stopped. The thyroid stimulating hormone level was 0.004 mIU/mL (normal: 0.35-4.8 mIU/mL), the free T4 level was 3.42 ng/dL (normal: 0.9-1.9 ng/dL). Thyroglobulin antibodies and Thyroid Peroxidase antibodies were elevated. A 24-hour radioiodine uptake measurement was elevated and a scan of the thyroid revealed diffuse elevated activity in the thyroid consistent with a diagnosis of Graves’ disease. The patient subsequently underwent radioiodine treatment of the thyroid gland.
Dr. Chu is a 3rd year resident in internal medicine at NYU Medical Center.
A PRACTICAL OVERVIEW OF THE IMMUNE THYROID DIATHESIS
Commentary by Manfred Blum MD, Professor, NYU Division of Endocrinology
Dr. Michael Chu has presented a classic instance of an allergic reaction to methimazole in a woman with thyrotoxic Graves’ disease and excellently reviewed the pathophysiology of the various thyroid antibodies. Let me briefly review the etiology, diagnosis and management of patients with the immune thyroid diathesis.
Immune thyroid disease (ITD) is responsible for the vast majority of hypo- and hyperthyroidism in the North America and for a much of euthyroid, thyroid enlargement. Worldwide, only iodine-deficiency accounts for more thyroid pathology.
ITD is not one disorder. Rather, there is a spectrum of clinical disorders that occur in a familial setting, most often among women, and frequently associated with other immune-mediated non-thyroid diseases. The disorders are not fixed and may wax and wane in intensity or evolve from one clinical expression to another, for instance from hyperthyroidism to hypothyroidism. ITD may be expressed solely in the thyroid, like goiter, have systemic secondary consequences, like myxedema, or primarily affect several organ systems, like Graves’ disease. The manifestations of ITD are influenced by co-morbidities, past events, environmental factors, and notably local T-lymphocyte phenomena and several immunoglobulins that have unique specificities and impact on thyroid function. TSI binds to and stimulates the TSH receptor to enhance thyroid hormone levels, anti-TPO antibody interferes with the function of the thyroid peroxidase enzyme to reduce thyroxine production, TSH-blocking antibody impedes TSH and TSI activity at the TSH receptor, also reducing hormone levels, but anti-thyroglobulin antibodies have no known hormonal consequences. All of the antibodies cross the placenta and affect the fetal and neonatal thyroid for the duration of maternal 7S immunoglobulins in the neonatal circulation. There are no easily available assessments of the T-lymphocyte factors and their pathophysiology is a subject of ongoing investigation.
The presence of anti-thyroid antibodies in the serum is a label signifying that autoimmune disease is operative and that other illness of this type may be present in the patient or family. The assays offer essential specific diagnostic information about etiology but no insight into thyroid function. The latter requires measurement of TSH.
The following table offers guidelines to common clinical use of anti-thyroid serologic tests.
Â
GUIDELINES TO THE USE OF ANTI-THYROID SEROLOGIC TESTS
Â
|
Clinical Condition |
Why Do Tests |
ATPO |
ATg |
TSI |
CONSIDER Next StepS |
| Goiter | Assess for AITD |
X |
X |
 |
If ITD (+), consider presence of other AIDs
Check TSH |
| Dominant Nodule in Goiter |
|
X |
X |
 |
If (+) consider other AIDs
If (+) check TSH
|
| Thyrotoxicosis
  |
Assess for ITD etiology
If positive    If negative |
X |
X |
X |
If (+) consider other AIDs
If (+) Graves’
 If (-) R/O TNG or TAN & Rx |
| Euthyroid Exophthalmos | Assess for ITD
If positive   If negative |
X |
X |
X |
Â
If (+) Graves’ eye disease & Monitor for hyperthyroidism If (+) consider other AIDs If (-) R/O local lesion |
| Hypothyroidism with high TSH | Assess for ITD etiology |
X |
X |
 |
If (+) Hashimoto’s & Rx with T-4
If (+) consider other AIs If (-) R/O other etiology |
| Hypothyroidism with low TSH | Â |
 |
 |
 |
Evaluate Pituitary |
| Family history of AI disorders | Assess for sub-clinical ITD |
X |
X |
 |
If (+) check TSH & thyroid size
If (-) Observe thyroid |
| Routine Screening | No |
 |
 |
 |
 |
ITD = immune thyroid diseases, AIDs = autoimmune diseases, ATPO = Antithyroid peroxidase antibodies, ATg = Antithyroglobulin antibodies, TSI = Thyroid stimulating immunoglobulin, FNB fine needle biopsy of thyroid nodule, Rx = treat
 References
1Adams DD, Fastier FN, Howie JB et al. Stimulation of the human thyroid by infusions of plasma containing LATS protector. J Clin Endocrinol Metab 1974; 39:826.
 2Hadj-Kacem H, Rebuffat S, Mnif-Feki M, Belguith-Maalej S, Ayadi H, Peraldi-Roux S. Autoimmune thyroid diseases: genetic susceptibility of thyroid-specific genes and thyroid autoantigens contributions. International Journal of Immunogenetics. 36(2):85-96, 2009 Apr.
 3Costagliola S, Morgenthaler NG, Hoermann R, Badenhoop K, Struck J, Freitag D, Poertl S, Weglohner W, Hollidt JM, Quadbeck B, Dumont JE, Schumm-Draeger PM, Bergmann A, Mann K, Vassart G, Usadel KH. Second generation assay for thyrotropin receptor antibodies has superior diagnostic sensitivity for Graves’ disease. J Clin Endocrinol Metab 1999 Jan;84(1):90-7.
 4Orgiazzi J. Anti-TSH receptor antibodies in clinical practice. Endocrinol Metab Clin North Am. 2000 Jun;29(2):339-55, vii.
 5Macchia E, Concetti R, Borgoni F, Cetani F, Fenzi GF, Pinchera A. Assays of TSH-receptor antibodies in 576 patients with various thyroid disorders: their incidence, significance and clinical usefulness. Autoimmunity. 1989;3(2):103-12.
 6Takasu N, Oshiro C, Akamine H, Komiya I, Nagata A, Sato Y, Yoshimura H, Ito K. Thyroid-stimulating antibody and TSH-binding inhibitor immunoglobulin in 277 Graves’ patients and in 686 normal subjects. Journal of Endocrinological Investigation. 20(8):452-61, 1997 Sep.
 7Wortsman J, McConnachie P, Tahara K, Kohn LD. Thyrotropin receptor epitopes recognized by Graves’ autoantibodies developing under immunosuppressive therapy. J Clin Endocrinol Metab. 1998; 83(7):2302-2308.
 8Chiovato L, Fiore E, Vitti P, et al. Outcome of thyroid function in Graves’ patients treated with radioiodine: Role of thyroid-stimulating and thyrotropin-blocking antibodies and of radioiodine-induced thyroid damage. J Clin Endocrinol Metab. 1998; 83:40-46.
 9Lab facets: Thyroid Stimulating Immunoglobulins (TSI). https://www.labcorp.com/pdf/TSI_Thyroid_Stimulating_Immunoglobulin_LabFacets_1442.pdf. Accessed 7/13/09.
 10Treatment Guidelines for Patients With Hyperthyroidism and Hypothyroidism. American Thyroid Association. 1995. http://www.thyroid.org/professionals/index.html.
 11Davies T. Pathogenesis of Hashimoto’s Thyroiditis (chronic autoimmune thyroiditis). Uptodate.com. Accessed 7/15/09.