Commentary by Elizabeth Hackett MD, PGY-3
On July 25th, 2007, the NYC Department of Health released an advisory requesting that all New York City physicians maintain a high index of suspicion for Legionnaires’ disease in patients presenting with community acquired pneumonia. This advisory was prompted by 27 cases of Legionella pneumonia reported in the Parkchester neighborhood of the Bronx during the fall of 2006 (zip code 10462 ). This cluster of cases represented an increase in incidence of the disease to 16.6 cases/100,000 in the Bronx, up from 2.3 cases/100,000 citywide.1
This increase in incidence set of an epidemiological quest for a common source for the outbreak, which has yet to be discovered. In order to assist the DOH in discovering and eradicating a source of this outbreak, they are requesting that we remain vigilant in testing for Legionnaires’ disease in our patients. Sputum or brochoalveolar lavage cultures are the preferred method of diagnosing Legionella pneumonia, with BAL being superior. The urine antigen test can be used as an adjunct, but should be accompanied by an attempt to validate the diagnosis by culture.
Legionnaires’ disease, of course, got its name from the 1976 outbreak during the Pennsylvania State American Legion Convention. 221 people were infected in the outbreak, thought to be caused by contaminated water in the hotel’s air-conditioning system, and 34 of them died. McDade and Shepard, 2 biologists working for the US Centers for Disease Control and Prevention, discovered that the causative agent was small fastidious gram negative rod, which they named after the unfortunate victims.3
This was not the first outbreak of Legionella. Once the pathogen was identified, antibody testing showed that several outbreaks of pneumonia in the 1950s and 60s were also likely caused by Legionella species. In fact, it was subsequently discovered that a six-year long epidemic of Legionnaires’ disease was ongoing among British tourists staying at one particular hotel in Spain, which leads me to wonder about the common sense of British tourists.3
Legionella bacteria are naturally occurring aquatic bacteria that grow in warm water, particularly in cooling towers, water heaters and potable-water plumbing. They are obligate aerobes, and are best grown on BCYEa agar, growing in about 2-5 days in the lab. There are 49 different Legionella species, 20 of which have been reported to infect humans. In addition, there are at least 16 different serogroups. L. pneumophilia serogroup 1 caused the 1976 outbreak and is the cause of seventy to ninety percent of all cases where the bacteria have been isolated.2
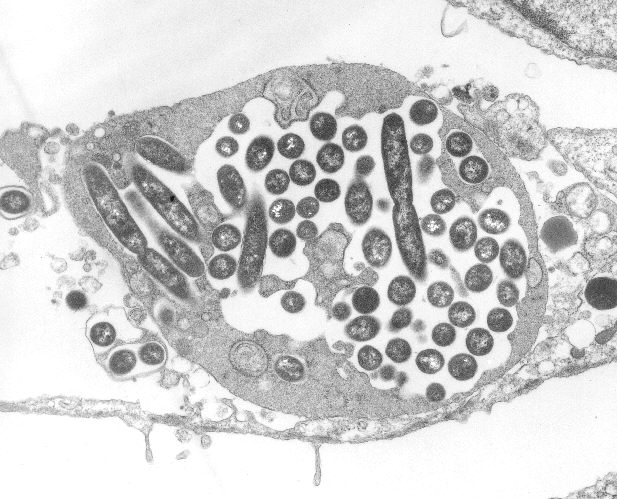
Hosts are infected when the bacteria are aerosolized from the contaminated water, inhaled by the host, or microaspirated. Once aspirated, in the lung, the bacteria begin their invasion. During the first stage, Legionella bacteria are phagocytosed by alveolar macrophages. After sufficient growth, the bacteria kill their macrophage host, escape into the extracellular environment and lie in waiting to be re-phagocytosed by another unsuspecting macrophage. After several rounds of this trick, the bacteria can increase their numbers ~100,000-fold over 3 days of initial infection (oh to be a unicellular organism!).2
Meanwhile, the hapless macrophages release the chemokines and cytokines of distress (including TNFa, IL-12, IL-18, IFNg), causing the usual cascade of neutrophil recruitment, infiltration of the alveoli, capillary leakage and edema, thus reeking havoc on the gas exchange of the lung. It is unknown how the bacteria evade neutrophils and the compliment system during this phase. Systemic spread my also occur, which is also likely accomplished by infection of circulating macrophages.3
Eventual control of the infection occurs when the cellular immune system, using TH1 (helper) T-cell responses. INFg allows macrophages to fend off L. pneumophilia growth, at least partially by reducing intracellular iron, which is required for the bacteria to replicate. The humoral response has not been shown to play a role in controlling Legionella, and previous infection does not necessarily confer immunity on the host.3
Clinical features of Legionnaires’ disease include an incubation period of 2-10 days (medium 4-6 days in several studies). Person to person transmission does not occur. Sixty-five to seventy-five percent of all reported cases are sporadic, that is, not associated with a known epidemic. The disease is likely underreported due to poor sensitivity of diagnostic testing. There are 0.4/100,000 cases per year reported in the US. However, prospective studies have estimated cases to represent 0.5 to 10% of all cases of community acquired pneumonia admitted to hospitals.3 Mortality rates are estimated to be 10 to 15%, compared with 5% of combined ambulatory and hospitalized patients with community acquired pneumonia, and 36% of patients with CAP admitted to the ICU.
Risk factors for developing Legionnaires’ disease include male sex, cigarette smoking, chronic heart or lung disease, ESRD, and age greater than 50 years. Additionally, recent travel, well water in the home, recent plumbing work, older plumbing, electric water heaters, whirlpool spas, hot water spring spas, decorative fountains and close proximity to cooling towers conferred greater risk on patients. Nosocomial Legionella pneumonia is thought to be caused by nebulizers, humidifiers, respiratory ventilators, lavage equipment, and NG tubes lavaged with tap water (no hospital procedure, no matter how benign, is without risk!).3
Legionella species cause an acute consolidating pneumonia, radiographically similar to pneumococcal pneumonia. Distinctive features include fever, with pulse-temperature dissociation (now thought to be less common than previously reported, but more common in elderly patients with Legionnaires’disease), myalgia, nonproductive cough, diarrhea, confusion, hyponatremia, elevated LFTs, and hypophosphatemia. However, these findings are too infrequent to differentiate Legionella from other causes of community acquired pneumonia and clinical scoring systems have failed to provide adequate sensitivity or specificity to be a useful diagnostic adjunct.3
Other less common symptoms include severe headache with obdundation, seizures, and focal neurological findings, and pleuritic chest pain with or without hemoptysis, which may confuse the diagnosis with PE or pulmonary infarct. Immunocompromised hosts may develop cavatery lung lesions. Other laboratory findings include hyperbilirubinemia, thrombocytopenia, leukopenia or leukocytosis, pyuria, increased CK (MM fraction), increased LDH, elevated serum creatinine with urinary casts, white cells and myoglobinuria. Hypoxemia is seen in proportion to severity of consolidation.3
Because Legionella species are intracellular pathogens residing inside of macrophages, they must be treated with antibiotics that are active within these cells. Macrolides such as azythromycin, and quinolones are the treatment of choice. Tetracyclines are also likely to be effective. Beta-lactam antibiotics are not effective for this disease.3
Laboratory tests for Legionella are generally specific, but lack sensitivity. Cultures of respiratory tract secretions are 100% specific, but only 20-95% sensitive, with sensitivity improving with severity of disease. Similarly the urine antigen is >99% specific, but 60-95% sensitive. The urine antigen tests only for L. pneumophilia serotype 1, which accounts for 70-90% of cases, thus missing 10-30% of active infections. Immunoflourescent microscopy has similar test performance as culture, and requires specially trained personnel. PCR is only available in experimental laboratories, and has a similar sensitivity to traditional culture at a significantly greater expense. Serologic tests are not usually useful in the clinical setting, but can be done if cultures and urine antigen testing are negative, but a strong clinical suspicion remains. Titers are drawn in paired serum samples during active and convalescent phases of the disease (3-4 weeks apart). A fourfold rise in titer is suggestive. IgG and IgM titers should be tested, as some individuals do not mount an IgG response.4
Please keep this diagnosis in mind and resist the temptation just to treat without testing for Legionella. Adequate testing and reporting may help eradicate an ongoing environmental source.
References:
1. Sharon Balter, M.D. New York City Department of Health and Mental Hygene. Revised DOHMH Advisory #2. July, 2997
2. Fields BS, Benson RF, Besser PR. Legionella and Legionnaires’ Disease: 25 years of investigation. Clin. Microb. Rev. 2002 Jul 15(3)506-26.
3. Mandell, Douglas, and Bennett. Principles & Practice of Infectious Diseases. New York : Elsevier/Churchill Livingstone. 2005.
4. Stout, JE, and Yu, VL. Legionellosis. NEJM. 337(10):682-687. Sept. 1997.
Image courtesy of Wikimedia Commons, Transmission electron micrograph of Legionella pneumophila multiplying inside a cultured human lung fibroblast.