Faculty Peer Reviewed
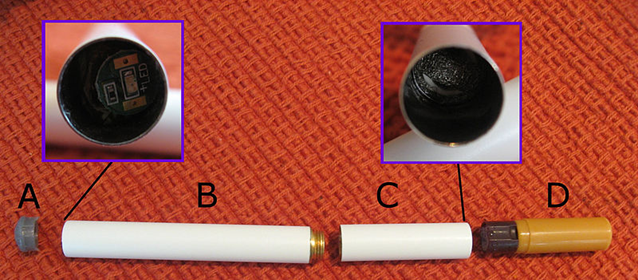
Image. Typical e-cigarette consisting of (A) LED light, (B) battery/circuitry, (C) atomizer, (D) replaceable mouthpiece cartridge containing glycerin or propylene glycol, water, flavorings, and often dissolved nicotine. Courtesy of Horsten at en.wikipedia.
A man sits in a crowded Manhattan coffee shop, enjoying his oversized latte while reading his favorite blog on an ultrathin laptop. There are several devices plugged into the computer’s USB ports: an external hard drive, a smart phone, and… a cigarette? He unplugs the cigarette, takes a deep inhalation, and with a satisfied expression exhales a cloud of mist that resembles cigarette smoke, but is nearly odorless and dissipates almost immediately. Another patron of the coffee shop turns around and provides the smoker with an undeniable look of disgust and anger. The smoker replies, “Relax, it’s just vapor, and it’s helping me quit those awful cancer-sticks that I’ve been hooked on for years.”
An electronic cigarette, or e-cigarette, is a battery-operated device that looks strikingly similar to a traditional cigarette. When the user inhales, air is propelled through a heated cartridge containing liquid nicotine, flavoring, and a variety of other chemicals. These substances vaporize and are transported into the user’s lungs. The process closely mimics the act of smoking a traditional cigarette, except that the “smokeless” aspect makes public use acceptable in many circumstances. This versatility, along with the device’s low cost, wide availability, and ease of use are likely reasons for the recent explosion of e-cigarette use in the United States. In fact, web-survey data recently published by the Centers of Disease Control and Prevention demonstrate that the percentage of smokers who have tried e-cigarettes more than doubled from 2010-2011, from 9.8%-21.2% [1]. As expected, government regulatory agencies and the medical community have become engaged in a debate regarding the health effects of these devices and their potential role in smoking cessation.
The Food and Drug Administration (FDA) has taken a strong stance against e-cigarettes. The organization’s initial attempt to tightly regulate the product as a pharmaceutical device was blocked by the US Court of Appeals in 2010, since e-cigarettes are not marketed for smoking cessation. However, the FDA was granted permission to regulate e-cigarettes as it would other tobacco products, and it is expected to make a statement in the near future regarding new regulations. In the meantime, the FDA has issued reports advising consumers of the dangers of the product based on their analysis of cartridges from two leading brands. These studies reveled carcinogenic nitrosamines to be present in at least half of the samples tested, and other carcinogenic impurities such as anabasine, myosmine and ?-nicotyrine in the majority of samples [2]. They also found diethylene glycol, a toxic ingredient in anti-freeze, to be present in one cartridge. Finally, they determined that “quality control processes used to manufacture these products are inconsistent or non-existent” [2].
In addition to concerns about the materials used in the product itself, researchers are also wary about the implications that e-cigarettes may have on smoking behaviors. In an editorial published in the New England Journal of Medicine in 2011, the authors expressed concern that e-cigarettes could easily become “bridge products” that smokers could use to quell nicotine cravings in settings where traditional cigarettes are banned [3]. Additionally, they could be used by non-smokers or former smokers as “starter products” that could lead to nicotine addiction and traditional cigarette use [3]. There are some data to back-up this concern: a 2010 online survey found that 2.0% of former smokers and 0.8% of never-smokers have tried e-cigarettes [4]. Furthermore, the variety of flavors that are offered make these products especially appealing to young people. Additionally, the cost of e-cigarettes is often considerably less than traditional cigarettes. Finally, there is concern that e-cigarettes may be used by consumers for smoking cessation in place of existing FDA-products that have already been proven safe and effective, but are more expensive.
Proponents of e-cigarettes point to studies that claim to demonstrate the product’s use as a smoking cessation device. In a recently published prospective study conducted in Italy, 300 traditional cigarette smokers were provided with a 12-week supply of electronic cigarettes [5]. These individuals had no intention of quitting when they enrolled in the study, but 52 weeks after the start of the study 8.7% had successfully quit smoking traditional cigarettes, compared to the 0.02% yearly average cessation rate in Italy [5]. While the study demonstrates that e-cigarettes may have a potential role in smoking cessation, it does not evaluate their long-term safety or compare their effectiveness to other smoking cessation devices. Additionally, there is no group that serves as a control for the use of e-cigarettes, which could introduce an element of sampling bias. Finally, 26.9% of traditional cigarette quitters continued to use e-cigarettes at 52 weeks, and an unknown proportion of subjects continued to use both traditional and electronic cigarettes [5].
E-cigarette proponents assume that replacing traditional cigarettes with e-cigarettes is safer for users and those around them. They may cite the fact that the carcinogens found in the FDA’s analysis were present at levels only 0.07–0.2% of those found in traditional cigarettes [6]. However, these studies examine only a portion of the available e-cigarettes on the market. The growing variability in brands and lack of quality control make it impossible to make a general statement regarding the overall safety of e-cigarettes. Additionally, randomized placebo-controlled trials assessing the long-term safety of e-cigarettes are non-existent. Although the aforementioned Italian study attempts to demonstrate the safety of these products by showing study-long reductions in adverse events such as “dry cough” [5], this more likely stems from reductions in traditional cigarette use and does not relate to the safety of e-cigarettes themselves. In contrast, the efficacy and safety of current FDA-approved smoking cessation devices have been studied extensively [7]. Unfortunately, clinicians will probably remain ignorant about the overall safety of e-cigarettes, as reliable data will only emerge if a pharmaceutical company is willing to produce an e-cigarette that is marketed for smoking cessation. Only then will it fully be evaluated by the FDA as a drug and drug-delivery device.
Clinical Bottom Line
– E-cigarettes are already widely available and their prevalence is increasing [1].
– E-cigarettes contain varying amounts of nicotine, and many of the same carcinogens as traditional cigarettes [2].
– There is considerable variability within and between brands in terms of carcinogen and nicotine content [2].
– While studies have demonstrated lower carcinogen levels in e-cigarettes compared to traditional cigarettes [6], quality data from animals and humans addressing long-term safety are very limited.
– While they may hold promise as smoking cessation devices in the future [5], e-cigarettes should not yet be recommended over products that are proven safe and effective such as patches, gum, lozenges, and inhalers.
Daniel Taupin is a 4th year medical student at NYU Langone Medical Center
Peer reviewed by Ellie Grossman, MD, NYU Langone Medical Center
References
1. King BA, Alam S, Promoff G, Arrazola R, Dube SR. Awareness and ever use of electronic cigarettes among U.S. adults, 2010-2011. Nicotine Tob Res. 2013;28:28. https://www.ncbi.nlm.nih.gov/m/pubmed/23449421/?i=6&from=/23741939/related
2. U. S. Food and Drug Administration. Summary of results: laboratory analysis of electronic cigarettes conducted by FDA. http://www.fda.gov/NewsEvents/PublicHealthFocus/ucm173146.htm. Updated July 22, 2009. Accessed August 21, 2012.
3. Cobb NK, Abrams DB. E-cigarette or drug-delivery device? Regulating novel nicotine products. N Engl J Med. 2011;365(3):193-195. http://www.ncbi.nlm.nih.gov/pubmed/21774706
4. Pearson JL, Richardson A, Niaura RS, Vallone DM, Abrams DB. e-Cigarette awareness, use, and harm perceptions in US adults. Am J Public Health. 2012;102(9):1758-1766. http://www.ncbi.nlm.nih.gov/pubmed/22813087
5. Caponnetto P, Campagna D, Cibella F, et al. EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PloS one. 2013;8(6). http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0066317
6. Wagener TL, Siegel M, Borrelli B. Electronic cigarettes: achieving a balanced perspective. Addiction. 2012;107(9):1545-1548. http://www.ncbi.nlm.nih.gov/pubmed/22471757
7. Moore D, Aveyard P, Connock M, Wang D, Fry-Smith A, Barton P. Effectiveness and safety of nicotine replacement therapy assisted reduction to stop smoking: systematic review and meta-analysis. BMJ. 2009;2(338):b1024. http://www.bmj.com/content/338/bmj.b1024

2 comments on “Electronic Cigarettes: What We Know So Far”
Thank you for this article. There is another really crucial study that has just been presented by Doctor Farsalinos at ESC Congree (see here : Doctor Farsalinos website)
and Press releas publish on theheart.org
and for French spoken people here : Cigarette Electronique
It’s really crucial because contrary to public perception, smoking-caused heart disease actually results in more deaths per year than smoking-caused lung cancer
Anecdotal evidence would suggest that these devices do little to help people quit. I’ve encountered many people who claim the devices have not helped quit smoking but have simple become a nicotine delivery system when they are in places that they cannot smoke.
Comments are closed.