 Commentary by Daniel Frenkel, MD PGY-2 and Aleksandar Adzic, MD PGY-2 (in consultation with Greg Mints, MD Attending Physician, General Internal Medicine)
Commentary by Daniel Frenkel, MD PGY-2 and Aleksandar Adzic, MD PGY-2 (in consultation with Greg Mints, MD Attending Physician, General Internal Medicine)
Case #1: A 47 year old man with no significant medical history, nonsmoker, and no family history of CAD. Blood pressure 124/72 Cholesterol 202, LDL 129, HDL 35, Triglycerides 190.
Case #2: A 36 year old man history of hypertension controlled with hydrochlorothiazide, smoker, with no family history of CAD. Blood pressure 134/72 Cholesterol 168, LDL 91, HDL 46, Triglycerides 155.
Would you prescribe daily Aspirin to either of these individuals in clinic?
Cardiovascular disease (CVD) defined as coronary heart disease, stroke, or peripheral vascular disease is the leading cause of death in the developed world accounting for approximately 900,000 deaths annually in the U.S. Numerous trials have demonstrated the benefits of aspirin including decreasing risk of myocardial infarction, stroke, and vascular mortality in acute coronary syndromes, acute occlusive stroke, and secondary prevention post MI, stroke, TIA, stable angina, or CABG. However, in patients without known cardiovascular disease, when should primary care physicians prescribe daily aspirin? To help answer this question there are 5 major randomized clinical trials that are the basis of the current guidelines.
The first 2 major randomized trials were the Physician’s Health Study (PHS) and British Doctor’s Trial (BDT) from the late 1980’s. The BDT had conflicting results with the PHS. The PHS, which was terminated early due to “extreme benefit,†showed significant reduction in MI but no difference in CVD mortality or stroke. However, the BDT showed no difference in any end point. Interpreting these results was difficult as both were affected by selection bias given that subjects were physicians, undermining the applicability of the results. The BDT specifically had no placebo control (“placebo†group instructed to avoid aspirin), no blinding, and roughly 30% in the aspirin group withdrew secondary to GI side effects. One could also question whether this study was powered to detect a significant difference given a much lower subject population.
The other 3 major randomized trials, published 10-15 years later,  included the Thrombosis Prevention Trial (TPT), the Hypertension Optimal Treatment Trial (HOT), and the Primary Prevention Project (PPP). These trials assessed the effects of aspirin in higher risk patients without known CVD and consistently demonstrated a significant reduction in major CVD events, specifically nonfatal ischemic heart disease. However, the TPT and HOT showed no difference in CVD mortality or total mortality. The PPP trial did demonstrate a difference in CVD mortality but no difference in total mortality. Unfortunately, the PPP trial was open label, making it less likely to affect an end point such as mortality. In addition, it was terminated early when the TPT and HOT results were published which may have biased the results, especially since it has been the only major trial to conclude a mortality benefit. The HOT trial showed a significant relative risk increase in hemorrhagic strokes while the other two trials did not demonstrate this difference but did show an increase in major bleeds.
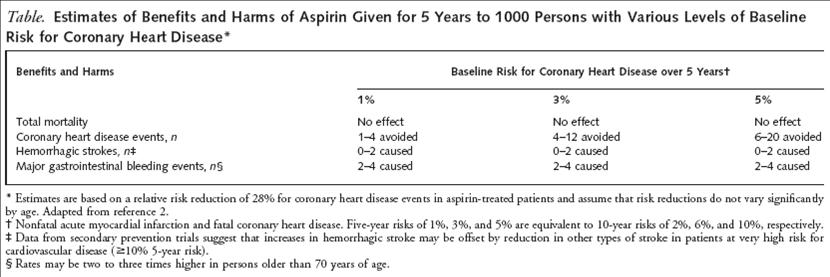
In 2003, a meta-analysis of these five major trials was presented in the Archives of Internal Medicine which compiled the data and demonstrated a significant 32% RRR in first MI and a 15% RRR of any important vascular event. Clearly daily aspirin has some benefit, but what threshold should be used to initiate primary prevention? One of the most widely used risk assessment tools for cardiovascular disease is the Framingham Risk Score. The American Heart Association suggests initiating daily aspirin when a patient’s 10-year risk score predicts at least 10% risk of cardiovascular disease while the United States Preventive Services Task Force (USPSTF) recommends using a cut-off of at least 6% because this is the threshold when the benefits (i.e. reduction in mainly nonfatal MI) outweighed the risks (i.e. increase major GI or CNS bleed) according to their own meta-analysis.
 Â
Â
USPSTF. Annals of Internal Medicine. 2002. 136: 157-172.
If we were to apply these guidelines, we would find that both cases #1 and #2 from the beginning of this post have 10-year CVD risk ≥ 6% (case #1: Framingham score 10 = 6% risk, Case #2: Framingham score 11 = 8% risk) so based upon the USPSTF cut-off we could initiate treatment with 81mg of aspirin daily. This all sounds easy and simple but if one were to take a step back there are few points to consider. 1) Most of the data from the trials used to determine these guidelines were not done in the era of statins, and one could question how much impact aspirin might have in this setting. 2) In calculating the benefit-risk analysis, the USPSTF assumed that risk of bleeding was constant across varying CVD risks, but if CVD risk goes up with age, doesn’t the risk of bleeding? 3) Several of the trials had been terminated early based upon prior results suggesting an overwhelming benefit from aspirin. However, it has been noted that many trials that are terminated early often have an overestimation of the benefit of an intervention especially when not enough data points are collected. This bias is perpetuated in the meta-analyses that use these trials in their calculations. 4) Is using the Framingham Risk Score an ideal risk assessment tool? The risk score actually attempts to predict risk of angina, MI, or coronary disease death. If we were using this risk assessment tool to assess high risk we may be considering many patients that simply develop angina as high risk. Should those patient receive aspirin daily and reduce the risk of a nonfatal symptom while putting them at risk for hemorrhage? Furthermore, is the Framingham Risk Score able to predict patients at high lifetime risk if that risk will not manifest in the next 10 years and more importantly, if we could identify those patients should they be considered for primary prevention with aspirin?
Despite these shortcomings the best model we currently have is the Framingham Risk Score as an adjunct to assess those at highest risk for CVD, but it should not replace clinical judgment. The current guidelines ultimately recommend daily aspirin for primary prevention be entertained in men or women at higher risk for CVD and that the benefits and risks be discussed on an individual basis. For example, those at risk for hemorrhage, whether cerebral or gastrointestinal, would clearly fall into the group of patients that one might not consider aspirin for primary prevention regardless of CVD risk.
But that’s not the end of the story. Most of the data used to synthesize these guidelines did not address whether they would apply to women or diabetics. The HOT and PPP trials were the only 2 of the previously mentioned trials that included women while the Women’s Health Study was a large randomized, double-blind, placebo controlled trial of 39,876 females, >45 years old, followed for about 10 years, that attempted to address this issue. The results demonstrated a significant reduction in total stroke and ischemic stroke but no difference in first major CVD event, hemorrhagic stroke, total MI, or CVD mortality. There was a significant increase risk of GI bleeding (RR 1.4). Similar to all the previously mentioned trials there was no significant change in mortality. However, instead of a reduction in nonfatal MI, there was a reduction in rates of stroke. The current guidelines intend to treat women the same as men and similarly use the Framingham Risk Score to assess CVD risk, but is this appropriate?
Diabetic patients are sometimes referred to as “CAD equivalent†and as such considered high risk (i.e. >20% 10-year CVD risk). With that in mind should 25 year old diabetics automatically be considered high risk and be given daily aspirin for primary prevention? The current guideline by the American Diabetic Association suggests daily aspirin for diabetics at higher risk for CVD, such as >40 years old, family history, hypertension, smoking, dyslipidemia, albuminuria. Between the age of 30-40 there is no clear answer, and those under 30 years of age have never been studied. More specifically the ADA does not recommended aspirin in patients <30 years of age because of the risk of Reye’s Syndrome. Ideally we should probably treat diabetics just as we do other patients and risk stratify them since not all diabetics are at equivalent risk. The current Framingham Risk Score does not take into account diabetes as a risk factor and may not be as reliable so a more specific cardiac risk assessment tool for type II diabetics without known disease has been developed known as the UKPDS Risk Engine.
Take-Home Points:
In patients without known cardiovascular disease, aspirin provides a statistically significant reduction in nonfatal MI but there is not enough evidence to conclude any effects on stroke and mortality. The benefits seem to outweigh risks with 10-year CVD risk of ≥ 6%, however, the initiation of daily aspirin and benefit-risk assessment should be discussed with every individual.
REFERENCES:
Physicians’ Health Study. New England Journal of Medicine. 1988; 318: 262-264.
Peto R., et al. British Medical journal. 1988; 296: 313-316.
Meade T.W., et al. Lancet. 1998; 351: 233-241.
Hansson L., et al. Lancet. 1998; 351: 1755-1762.
De Gaetano., et al. Lancet. 2001; 357(9250):89-95.
Eidelman R. S., et al. Archives of Internal Medicine. 2003; 163: 2006-2010.
USPSTF. Annals of Internal Medicine. 2002; 136: 157-172.
Ridker P. M., et al.. New England Journal of Medicine. 2005; 352: 1293-1304.
American Diabetes Association. Diabetes Care. 2007; 30(Suppl 1): S4-S41.
Mueller P.S., et al. Annals of Internal Medicine. 2007; 146: 878-881.

2 comments on “Aspirin Use in the Primary Prevention of Cardiovascular Disease”
nice ,brief review….. with authentic references….
Comments are closed.