Peer Reviewed
Stroke is among the costliest disorders in the world for both individuals and society. Every hour of an evolving stroke kills 120 million neurons, destroys 830 billion synapses and degrades 714 kilometers of myelinated fibers, aging the brain by 3.6 years in those 60 minutes1. It is the leading cause of adult disability in the USA, currently costing $70 billion a year2 with $2.2 trillion more projected over the next forty years3. The global burden is even higher.
Despite this increasingly severe problem, our approaches to the treatment of acute ischemic stroke (AIS) have changed remarkably little since the introduction of tissue plasminogen activator (tPA) in the mid-1990s4. Yet we are in the midst of a veritable renaissance in the management of AIS, with communities and clinicians implementing innovative ways of earlier detection and intervention, new pharmaceuticals for both thrombolysis and neuroprotection, improved stenting and clot dispersal devices, and more streamlined critical care after an ischemic event. Together, these new technologies offer hope for stroke patients and may return quality of life and productive years to both individuals and society.
The mantra of “time is brain†is entirely accurate in the management of AIS, and so shortening the time from detection to treatment is critical to improve outcomes. To this point, community outreach efforts to improve recognition, response to and understanding of stroke have been promising. The “Hip Hop Stroke†initiative, for example, seeks to teach school children the cardinal signs of stroke and how to immediately respond to them via interactive song and game based didactics, and then communicate that information to parents via homework and other activities5-7. Using this approach with a sample of 182 children in New York City, rates of recognition of the cardinal signs of stroke, along with knowledge of an action plan in response to them, went up from less than 4% to nearly 30% among families5.
The Hip Hop Stroke program and other similar initiatives hope to significantly decrease the time between stroke onset and intervention by empowering the community. These efforts are critical, as many patients currently arrive to the ED after the 3-4.5 hour window for tPA8,9, and while door-to-needle time can be reduced to 20 minutes in a well run stroke center10, patients often wait far longer for evaluation and treatment after arrival. There are several approaches being taken to reduce such time, including improved stroke triage education, EMS bringing stroke patients “straight to CTâ€, telestroke evaluation, and mobile stroke units that begin treatment before the patient even arrives to the hospital.
Multiple centers in the past few years have begun to mobilize specialized ambulances—“mobile stroke units,†or MSUs–with a full suite of equipment for diagnosis and treatment of stroke11,12. A recently published 21 month study equipped ambulances with an onboard CT scanner, blood testing (for INR, CBC and glucose), stroke-trained neurologist, radiology technician, and paramedic, allowing the start of tPA therapy en-route to a hospital13. The authors report that the tPA treatment rate increased by 50%, from 21.2% to 32.6% with these specialized ambulances. This may have come down to the alarm-to-treatment time being 25 minutes shorter with the MSU, allowing 58% of the MSU patients, versus 37% of controls, to be treated within 90 minutes of symptom onset. This mobile approach, however, is not perfect. Investigators found no significant difference in rates of ICH or death, and the scalability may be limited by cost; each unit costs approximately $1.4 million to outfit, and having a stroke-trained neurologist, radiographic technician and more on call for such ambulances may not be realistic for many areas.
In smaller or rural hospitals, or even overcrowded urban community centers, the issue may not be arrival time or nursing evaluation, but availability of stroke-trained physicians. Therefore, telestroke evaluation is a promising avenue. According to a study of smaller urban hospitals in California, by using telestroke with a stroke faculty at a larger center, the median time from initial ED call to stroke consult was 5 minutes, with the average tele-exam taking 30 minutes. With such expediency, tPA use rate increased between 2 and 6 times the normal at these hospitals, with limited burden on the off-site faculty14.
Once a diagnosis of acute ischemic stroke is confirmed, therapy has traditionally relied on pharmaceutical intervention. This focus goes back to 1996, when the NINDS-2 trial led to FDA approval for t-PA for acute ischemic stroke if symptom onset was within a 3-hour window. Patients in the study received 0.9mg/kg with 10% bolused and the rest given over the following 60 minutes4. Since that time, most major stroke centers have extended the time window from 3 to 4.5 hours, but even this extension fails to capture many stroke patients (significant limitations remain for diabetics and those over 80 years of age, a large proportion of the population at risk for stroke15) and the utility of tPA is diminished with each passing minute. In addition, a minority of patients even arrive to the ED in time to receive tPA16. Aside from these logistical issues, there are physiologic limitations to tPA. It often fails to cause rapid reperfusion and puts patients at increased risk of bleeding. A less discussed issue is the neurotoxicity that seems to be associated with its use, possibly due to blood-brain-barrier disturbance and interaction with NMDA receptors17. Therefore, improvements in drug efficacy, the therapeutic window and post-administration toxicity are essential to improving patient outcomes.
For the past decade, a wide range of alternatives to tPA have been sought, but thus far only tenecteplase and desmoteplase show clinical promise. First described in 1995 18, tenecteplase was not studied in the context of AIS until 200519. While this first study was stopped prematurely when doses over 0.5mg were found to cause increased risk of ICH, it was later shown that 0.25mg/kg of tenecteplase was superior to standard tPA per 24 hour and 90 day outcomes (72% showed excellent recovery at 90 days in the tenecteplase group, vs 44% in the tPA group), with no difference in bleeding risk or other complications and with a longer treatment window20 21. With such a promising set of studies, a phase III trial for the use of TNK in AIS in now in progress.
Perhaps even more promising, however, is desmoteplase, a compound first isolated from vampire bat saliva in 199122. It has a theoretical affinity for fibrin nearly 200 times higher than that of tPA23, and several studies have shown both its comparative efficacy and safety24,25, with a lower rate of neurotoxic complications than tPA26. The benefit over tPA may be higher for patients with severe rather than mild/moderate stenosis27, but phase III and IV studies are now ongoing regarding a single 90microgram/kg bolus of desmoteplase within a 3-9 hour window for treatment of AIS28.
Aside from pharmaceutical innovation, the past few years have seen substantial progress in novel devices that allow for localized administration of thrombolytics, stenting, and mechanical removal of a clot. In the early 2000s a series of studies initially suggested such interventions were ineffective or harmful. More recent trials, however, provide strongly evidence that interventional approaches are safe, efficacious and superior to the use of tPA alone. Of these trials, the MR CLEAN stands out as perhaps the most influential.
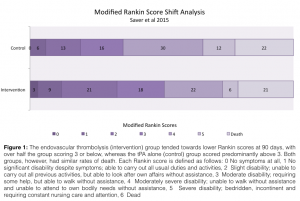
Enrolling 500 patients across the Netherlands, MR CLEAN29 randomly divided confirmed ischemic stroke patients into either a control group that received tPA alone or an interventional group that received intra-arterial treatment with either local delivery of tPA or urokinase (decision up to the provider), and/or mechanical thrombectomy with or without IV tPA. Inclusion criteria were relatively strict: eligibility required an NIHSS of >2 along with a CT, MRA or DSA-confirmed occlusion in a distal intracranial carotid artery, the MCA (M1 or M2), or the ACA (A1 or A2). Primary outcome was the modified Rankin scale score (mRSS) at 90 days, with secondary outcomes including the NIH Stroke Scale (NIHSS) at 24 hours and 5 or 7 days, or at discharge. By classifying a mRSS of 0-2 as a good outcome (indicative of functional independence after stroke) and a score of >3 as a poor outcome (functional dependence or death), the number needed treat for endovascular therapy to provide additional benefit over tPA alone was 5.3—a strikingly positive result. Aside from this primary outcome, the interventional group showed a positive “shift analysis†for mRSS, in which more patients scored lower on the scale than in the control group, as seen below. While the interventional group had a higher rate of new ischemic stroke in a different vascular territory (13/233 vs 1/267 in control), there was no difference in mortality at 7, 30 or 90 days, thus the authors concluded that treatment of proximal intracranial arterial occlusions within 6 hours of symptom onset by endovascular intervention leads to improved outcomes relative to tPA alone.
Since MR CLEAN, the SWIFT-PRIME, ACCORD, and REVASCAT trials have all shown similar efficacy to interventional thrombolysis over tPA alone, prompting the AHA to update their official guidelines for management of acute ischemic stroke to include endovascular procedures30. What changed, then, between the original negative studies on this topic and these newer positive ones? The answer likely lies in both study design and technology used. Earlier trials had limitations in design such as an overly long interval before treatment, absence of pretreatment imaging to confirm a proximal occlusion, and older, less nuanced devices. The IMS-III trial, for example, the most recent of the negative studies, did not use CT imaging to select patients appropriate for thrombolytic intervention until nearly 300 participants had been randomized, and used a smaller dose of tPA in the interventional group than the control for most of the participants.
Newer interventional devices also offer improved efficacy and safety through debulking, aspiration and direct extraction of a thrombus with a lower risk for clot displacement. The Solitaire Flow Restoration Device, for instance, has a 90-day mortality odds ratio of 0.34 compared to older devices such as the MERCI31. Others, like the Penumbra device, show similar improvements in efficacy per improvement on the NIHSS at discharge and 30-day modified Rankin scores32. Complication rates, including vasospasm, ICH and stroke in a new territory, are similar to current pharmaceutical therapy. In short, these new devices offer superior clot retrieval potential with similar or less risk than prior tools.
These advances in treatment options offer significant hope for improvement of patient outcomes, but also make selection of patients for a particular treatment approach all the more important. It has been well documented, for instance, that tPA is most effective in distal intracranial occlusions whereas intra-arterial thrombolysis is more effective in proximal occlusions with ischemic areas less than a certain size33,34. As such, improved imaging technologies may help to stratify patients and improve the outcome of those for whom treatment would be beneficial, while preventing unnecessary procedures and complications in those for whom it would not. While there is much on the horizon, however, for now the current AIS guidelines for imaging of stroke remain quite simple: non-contrast head CT prior to any intervention, be it tPA alone or endovascular thrombolysis. Other modalities, such as MRI, MRA, or CT perfusion/diffusion weight imaging, require further study but promise to more effectively differentiate between the infarct core and the potentially salvageable tissue around the core, termed the penumbra. Of these methods, CT perfusion is already the most widely used, as it can quickly and accurately help to select patients for endovascular therapy beyond the recommended 4.5 hours post-symptom onset. CT perfusion relies on three variables to determine the core and penumbra of an ischemic stroke: mean transit time (MTT), cerebral blood flow (CBF) and cerebral blood volume (CBV). Both the infarct core and penumbra have a prolonged MTT, but the core has a significantly lower CBF and CBV, whereas the penumbra typically has only a marginally decreased CBF with a normal or somewhat elevated CBV (secondary to reactive vasodilation)35.
As interventions and selection for stroke treatment improve, it is equally important to prevent lasting neurologic damage during and after the infarct. The effort to prevent ischemic neurotoxicity in AIS has been extremely challenging. Traditional approaches have assumed glutamate toxicity to be the primary cause of neurotoxicity, and have targeted NMDA receptors to prevent the extracellular calcium buildup that leads to enzyme activation with reactive oxygen species proliferation and subsequent cell death. The problem with this approach is that glutamate release happens very early in the ischemic cascade, and thus NMDA antagonists must be given within 30 minutes of stroke onset, which is rarely achievable in clinical practice. Targeting NMDA receptors is made more complicated by the fact that they seem to have contrary effects depending on location– synaptic NMDA receptors cause calcium influx that make neurons more resistant to ischemia, but extrasynaptic NMDA stimulation initiates apoptotic pathways36.
Given these issues with targeting NMDA receptors, recent studies have shifted focus onto other targets, the most promising of which is TRPM7, a newly discovered Ca+ permeable channel that strongly influences cell survival, as both over and under-expression of the gene lead to cell death37. Oxidative stress, decreased pH, and increased extracellular calcium and magnesium—which all occur in cerebral ischemia—activate TRPM7 independent of glutamate, and in vitro studies suggest this activation plays a large role in oxygen/glucose-deprivation cell death36,37. Interestingly, in vivo rat studies show that TRPM7 suppression makes neurons more resistant to death, maintains normal structural integrity, and allows better functional outcomes in the presence of oxidative stress38. Still, while these findings are promising, current TRPM7 inhibitors are nonselective and further study is needed before any clinical trials are considered.
While pharmaceutical and endovascular treatments have improved, sonothrombolysis, or high-intensity focused ultrasound, might someday offer an alternative approach to AIS. It is theorized to either boost drug transport into the clot to facilitate enzymatic fibrinolysis causing capillary vasodilation and enhanced tissue perfusion, or cause acoustic cavitation of the clot to promote dissolution 39. Initial studies found it to enhance the effect of tPA without affecting clot degradation independently40,41. Recent meta-analyses, however, show an odds ratio of 2.95 for complete recanalization in 1-2 hours, and 2.20 for minimal lasting deficit 90 days post-stroke, relative to intervention without sonothrombolysis39,42. Injectable intravascular microbubbles may further enhance this by oscillating when exposed to an ultrasound beam, mechanically disrupting the clot tissue; thus far, microbubbles appears to double the efficacy of sonothrombolysis without increased bleeding or endothelial and extravascular damage42. The use of sonothrombolysis, however, is still highly experimental with many limitations and, given the proven efficacy of intravascular devices, may not ultimately be clinically relevant.
Aside from the topics discussed above, there are significant opportunities to improve stroke at the preventive level, as well as in the days to weeks after stroke via a neurological ICU and intensive rehabilitation. For the acute management, however, what is clear is that after nearly two decades of relative stagnancy in stroke treatment, major inroads are being made to ensure timely identification, stratification, and safe, effective treatment of ischemic stroke, allowing patients to retain as much of their life—in quality and in years—as possible.
Dr. David Valentine is an Internist at NYU Langone Medical Center
Peer reviewed by Aaron Lord, MD, department of neurology, NYU Langone Medical Center Â
Image courtesy of Wikimedia Commons
ReferencesÂ
- Saver JL. Time is brain – Quantified. Stroke. 2006;37:263-266. http://stroke.ahajournals.org/content/37/1/263.full.pdf
- Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129(3):399-410. http://www.ncbi.nlm.nih.gov/pubmed/24446411
- Brown DL, Boden-Albala B, Langa KM, et al. Projected costs of ischemic stroke in the United States. Neurology. 2006;67:1390-1395. http://graphics.tx.ovid.com.ezproxy.med.nyu.edu/ovftpdfs/FPDDNCJCBFIFLL00/fs047/ovft/live/gv031/00006114/00006114-200610240-00018.pdf
- Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333(24):1581-1587. http://www.ncbi.nlm.nih.gov/pubmed/7477192
- Williams O, DeSorbo A, Noble J, Gerin W. Child-Mediated Stroke Communication: findings from Hip Hop Stroke. Stroke. 2012;43(1):163-169. http://www.ncbi.nlm.nih.gov/pubmed/22033995,  http://stroke.ahajournals.org/content/43/1/163.full.pdf
- Williams O, DeSorbo A, Noble J, Shaffer M, Gerin W. Long-term learning of stroke knowledge among children in a high-risk community. Neurology. 2012;79(8):802-806. http://www.ncbi.nlm.nih.gov/pubmed/22875089 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3421152/pdf/znl802.pdf
- Williams O, Noble JM. ‘Hip-hop’ stroke: a stroke educational program for elementary school children living in a high-risk community. Stroke. 2008;39(10):2809-2816. http://www.ncbi.nlm.nih.gov/pubmed/18635851
http://stroke.ahajournals.org/content/39/10/2809.full.pdf
- Saver JL, Smith EE, Fonarow GC, et al. The “golden hour” and acute brain ischemia: Presenting features and lytic therapy in >30 000 patients arriving within 60 minutes of stroke onset. Stroke. 2010;41:1431-1439. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2909671/pdf/nihms213423.pdf
- Byrne B, O’Halloran P, Cardwell C. Accuracy of stroke diagnosis by registered nurses using the ROSIER tool compared to doctors using neurological assessment on a stroke unit: A prospective audit. International Journal of Nursing Studies. 2011;48:979-985. http://ac.els-cdn.com/S0020748911000356/1-s2.0-S0020748911000356-main.pdf?_tid=79ff9040-2aae-11e5-9e83-00000aacb35d&acdnat=1436936637_08bab8853bd258b9c488fedba1f82bbc
- Meretoja A, Strbian D, Mustanoja S, Tatlisumak T, Lindsberg PJ, Kaste M. Reducing in-hospital delay to 20 minutes in stroke thrombolysis. Neurology. 2012;79:306-313. http://graphics.tx.ovid.com.ezproxy.med.nyu.edu/ovftpdfs/FPDDNCJCBFIFLL00/fs047/ovft/live/gv024/00006114/00006114-201207240-00007.pdf
- Rajan S, Baraniuk S, Parker S, Wu T-C, Bowry R, Grotta JC. Implementing a Mobile Stroke Unit Program in the United States. JAMA Neurology. 2015;72:229. http://archneur.jamanetwork.com/article.aspx?articleid=2020709
- Parker SA, Bowry R, Wu T-C, et al. Establishing the First Mobile Stroke Unit in the United States. Stroke. 2015;46:1384-1391. http://stroke.ahajournals.org/content/46/5/1384.long
- Ebinger M, Winter B, Wendt M, et al. Effect of the use of ambulance-based thrombolysis on time to thrombolysis in acute ischemic stroke: a randomized clinical trial. JAMA : the journal of the American Medical Association. 2014;311:1622-1631. http://jama.jamanetwork.com/article.aspx?articleid=1861800
- Cutting S, Conners JJ, Lee VH, Song S, Prabhakaran S. Telestroke in an Urban Setting. Telemedicine journal and e-health : the official journal of the American Telemedicine Association. 2014;20:855-857. http://online.liebertpub.com/doi/abs/10.1089/tmj.2013.0348
- Röther J, Ford Ga, Thijs VNS. Thrombolytics in acute ischaemic stroke: historical perspective and future opportunities. Cerebrovascular diseases (Basel, Switzerland). 2013;35:313-319. http://www.karger.com/Article/Pdf/348705
- Qureshi AI, Kirmani JF, Sayed MA, et al. Time to hospital arrival, use of thrombolytics, and in-hospital outcomes in ischemic stroke. Neurology. 2005;64(12):2115-2120. http://www.ncbi.nlm.nih.gov/pubmed/15985583
- Micieli G, Marcheselli S, Tosi PA. Safety and efficacy of alteplase in the treatment of acute ischemic stroke. Vascular Health and Risk Management. 2009;5:397-409. http://www.dovepress.com/getfile.php?fileID=4763
- Benedict CR, Refino CJ, Keyt BA, et al. New variant of human tissue plasminogen activator (TPA) with enhanced efficacy and lower incidence of bleeding compared with recombinant human TPA. Circulation. 1995;92:3032-3040. http://www.ncbi.nlm.nih.gov/pubmed/7586274
- Haley EC, Lyden PD, Johnston KC, Hemmen TM. A pilot dose-escalation safety study of tenecteplase in acute ischemic stroke. Stroke. 2005;36:607-612. http://stroke.ahajournals.org/content/36/3/607.full.pdf
- Parsons M, Spratt N, Bivard A, et al. A Randomized Trial of Tenecteplase versus Alteplase for Acute Ischemic Stroke. New England Journal of Medicine. 2012;366:1099-1107. http://www.nejm.org/doi/full/10.1056/NEJMoa1109842
- Parsons MW, Miteff F, Bateman Ga, et al. Acute ischemic stroke: Imaging-guided tenecteplase treatment in an extended time window. Neurology. 2009;72:915-921. http://www.neurology.org/content/72/10/915
- Kratzschmar J, Haendler B, Langer G, et al. The plasminogen activator family from the salivary gland of the vampire bat Dew&us rotundus: cloning and expression. Gene. 1991;105:229-237. http://www.ncbi.nlm.nih.gov/pubmed/1937019
- Bringmann P, Gruber D, Liese a, et al. Structural features mediating fibrin selectivity of vampire bat plasminogen activators. Journal of Biological Chemistry. 1995;270:25596-25603. http://www.jbc.org/content/270/43/25596.full.pdf
- Furlan AJ, Eyding D, Albers GW, et al. Dose Escalation of Desmoteplase for Acute Ischemic Stroke (DEDAS): Evidence of safety and efficacy 3 to 9 hours after stroke onset. Stroke. 2006;37:1227-1231. http://stroke.ahajournals.org/content/37/5/1227.full.pdf
- Hacke W, Albers G, Al-Rawi Y, et al. The Desmoteplase in Acute Ischemic Stroke Trial (DIAS): A phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke. 2005;36:66-73. http://stroke.ahajournals.org/content/36/1/66.full.pdf
- Atalaya JL, Benchenane K, Castel H, Ali C, Petersen K-U, Vivien D. Desmoteplase (DSPA) does not interact with or cleave the NMDA receptor NR1 subunit: Possible molecular basis for improved tolerability in treatment of acute ischemic stroke. Journal of Cerebral Blood Flow & Metabolism. 2005;25:S578-S578. http://www.nature.com/jcbfm/journal/v25/n1s/full/9591524.0578a.html
- Fiebach JB, Al-Rawi Y, Wintermark M, et al. Vascular occlusion enables selecting acute ischemic stroke patients for treatment with desmoteplase. Stroke. 2012;43:1561-1566. http://stroke.ahajournals.org/content/43/6/1561.full.pdf
- von Kummer R, Albers GW, Mori E, et al. The Desmoteplase in Acute Ischemic Stroke (DIAS) clinical trial program. International Journal of Stroke. 2012;7:589-596. http://onlinelibrary.wiley.com/doi/10.1111/j.1747-4949.2012.00910.x/abstract
- Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11-20. http://www.ncbi.nlm.nih.gov/pubmed/25517348
http://www.nejm.org/doi/pdf/10.1056/NEJMoa1411587
- Powers WJ, Derdeyn CP, Biller J, et al. 2015 American Heart Association/American Stroke Association Focused Update of the 2013 Guidelines for the Early Management of Patients With Acute Ischemic Stroke Regarding Endovascular Treatment: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2015;46(10):3020-3035. http://www.ncbi.nlm.nih.gov/pubmed/26123479
- Saver JL, Jahan R, Levy EI, et al. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380(9849):1241-1249. http://www.ncbi.nlm.nih.gov/pubmed/22932715
- Po Sit S. The penumbra pivotal stroke trial: Safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009;40:2761-2768. http://stroke.ahajournals.org/content/40/8/2761.full.pdf
- Davalos A, Pereira VM, Chapot R, et al. Retrospective multicenter study of Solitaire FR for revascularization in the treatment of acute ischemic stroke. Stroke. 2012;43(10):2699-2705. http://www.ncbi.nlm.nih.gov/pubmed/22851547
- Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38(3):967-973. http://www.ncbi.nlm.nih.gov/pubmed/17272772
- Kurz KD, Ringstad G, Odland A, Advani R, Farbu E, Kurz MW. Radiological imaging in acute ischaemic stroke. Eur J Neurol. 2016;23 Suppl 1:8-17. http://www.ncbi.nlm.nih.gov/pubmed/26563093
- Bae CYJ, Sun H-S. Current understanding of TRPM7 pharmacology and drug development for stroke. Acta Pharmacologica Sinica. 2012;34:10-16. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4086489/pdf/aps201294a.pdf
- Aarts M, Iihara K, Wei WL, et al. A Key Role for TRPM7 Channels in Anoxic Neuronal Death. Cell. 2003;115:863-877. http://ac.els-cdn.com/S0092867403010171/1-s2.0-S0092867403010171-main.pdf?_tid=2f9401e4-2aae-11e5-9662-00000aacb35e&acdnat=1436936511_87e2710d71fa488d6a36ed60b75460bb
- Sun H-S, Jackson MF, Martin LJ, et al. Suppression of hippocampal TRPM7 protein prevents delayed neuronal death in brain ischemia. Nature neuroscience. 2009;12:1300-1307. http://www.ncbi.nlm.nih.gov/pubmed/19734892
http://www.nature.com/neuro/journal/v12/n10/pdf/nn.2395.pdf
- Saqqur M, Tsivgoulis G, Nicoli F, et al. The role of sonolysis and sonothrombolysis in acute ischemic stroke: a systematic review and meta-analysis of randomized controlled trials and case-control studies. J Neuroimaging. 2014;24(3):209-220. http://www.ncbi.nlm.nih.gov/pubmed/23607713
http://onlinelibrary.wiley.com/doi/10.1111/jon.12026/abstract
- Frenkel V, Oberoi J, Stone MJ, et al. Pulsed high-intensity focused ultrasound enhances thrombolysis in an in vitro model. Radiology. 2006;239(1):86-93. http://www.ncbi.nlm.nih.gov/pubmed/16493016
- Stone MJ, Frenkel V, Dromi S, et al. Pulsed-high intensity focused ultrasound enhanced tPA mediated thrombolysis in a novel in vivo clot model, a pilot study. Thromb Res. 2007;121(2):193-202. http://www.ncbi.nlm.nih.gov/pubmed/17481699
- Lapchak Pa, Kikuchi K, Butte P, Holscher T. Development of transcranial sonothrombolysis as an alternative stroke therapy: incremental scientific advances toward overcoming substantial barriers. Expert Rev Med Devices. 2013;10:201-213. http://informahealthcare.com/doi/abs/10.1586/erd.12.88