 By Mary C. Whitman, MD
By Mary C. Whitman, MD
Faculty Peer Reviewed
Proton pump inhibitors (PPIs) are generally considered “safe†medications[1] and are prescribed to over 100 million patients per year for a variety of indications, often for …

 By Mary C. Whitman, MD
By Mary C. Whitman, MD
Faculty Peer Reviewed
Proton pump inhibitors (PPIs) are generally considered “safe†medications[1] and are prescribed to over 100 million patients per year for a variety of indications, often for …
 Summary by Lakshmi Tummala, MD
Summary by Lakshmi Tummala, MD
The Medical Grand Rounds lecture on October 6, 2010 titled “Influenza 2010: Update after the 2009 Pandemic was presented by Dr. Alicia Fry, M.D., MPH an …
Dana Clutter, MD
Edited by Vivian Hayashi MD and Robert Smith MD, Mystery Quiz Section Editors
Faculty peer reviewed
The answer to the mystery quiz is bacillary angiomatosis (BA). BA is a disease that most frequently affects individuals infected with the human immunodeficiency virus (HIV) and typically …
Dana Clutter, MD
Edited by Vivian Hayashi MD and Robert Smith MD, Mystery Quiz Section Editors
A 25 year old woman infected with HIV presents to an HIV/AIDS clinic in Kampala, Uganda, for evaluation of cutaneous lesions on her face, arms and back. Aside from the disfiguring nature of her lesions, she …
 By David Hormozdi, MD
By David Hormozdi, MD
The weather outside may be cooling off but the debate surrounding lung cancer screening is heating up once again as preliminary results released from The National …
 By Jon-Emile Kenny, MD
By Jon-Emile Kenny, MD
Faculty Peer Reviewed
A 37-year-old man, with no past medical history and taking finasteride for male pattern baldness, is admitted to Medicine with profound lower …
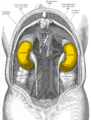 By Kevin Hsueh, MD
By Kevin Hsueh, MD
Faculty Peer Reviewed
In 2006 and 2007, Clinical Correlations reported on the FDA’s announcement of a link between Nephrogenic Systemic Fibrosis (NSF), a rare progressive …
 Nathaniel Rosso Smilowitz, MD
Nathaniel Rosso Smilowitz, MD
Faculty Peer Reviewed
Hepatitis B virus is a DNA hepadnavirus affecting 1.25 million people in the United States and nearly 400 million worldwide. The virus …