Faculty Peer Reviewed
Skin cancer is the most common type of cancer worldwide. In the United States, the incidence is rising, with over two million people diagnosed each year [1]. More cases of skin cancer are diagnosed each year than breast, prostate, lung, and colon cancer combined. The lifetime risk of developing skin cancer is estimated to be 20% [2]. Although nonmelanoma skin cancer is rarely fatal and associated with a very low mortality rate, melanoma can be highly fatal. Approximately 76,000 individuals will be diagnosed with invasive melanoma in 2012 [3]. Skin cancer can be easily cured with early detection and excision. Early detection is essential, especially for melanoma, which has a grim prognosis once it has metastasized. Today, detection requires a biopsy to definitively determine if a lesion is malignant or benign.
A skin biopsy is a simple procedure performed by a physician and takes only a few minutes. In general, it is quick and easy for the physician. From the patient’s standpoint, however, undergoing a skin biopsy can be an unpleasant experience. A biopsy can be painful and leaves behind an unwanted scar. The results of the biopsy can take up to weeks, keeping the patient in a seemingly endless state of worrying, especially when melanoma is suspected. Additional time accrues as the patient schedules and waits for a followup visit or consultation. On occasion, a repeat biopsy may even be needed. Overall, the patient undergoes a potentially painful, prolonged process with enduring effects.
Patients may someday have an alternative that is free of pain, waiting, and unnecessary scars. New technologies are emerging that may reduce the need for a skin biopsy. Noninvasive imaging technologies are able to look into the skin and detect malignancies in real time. The results are instantaneous. In addition, noninvasive imaging technologies preserve the integrity of the skin, allowing safe imaging of any aspect of a lesion as many times as needed. Images are collected and stored in digital format. Digital images are readily accessible and can be easily sent for referral or consultation, unlike conventional pathology slides. Noninvasive imaging modalities for the skin currently under investigation include confocal microscopy, optical coherence tomography, high-frequency ultrasound, and magnetic resonance imaging.
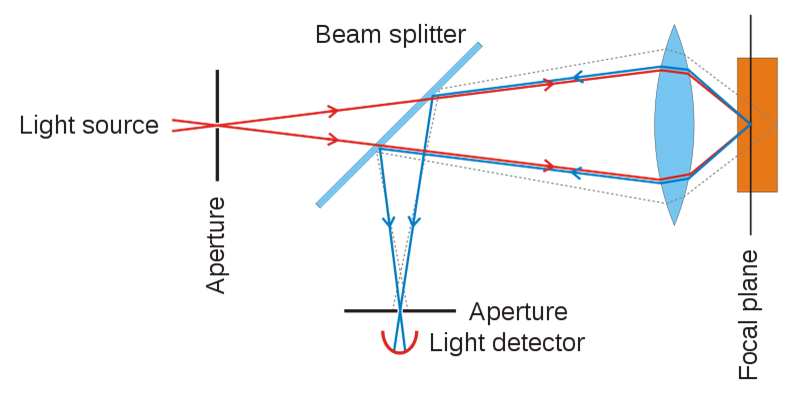
Confocal microscopy is the first imaging modality to offer single-cell resolution of the skin in vivo [4]. Confocal microscopy uses a focused laser beam to illuminate a specific point within the skin and measures the reflection of light from that point. Multiple points are scanned across an area parallel to the skin surface to construct a grayscale image. Various depths can also be imaged to form optical sections. Images can be produced with lateral resolutions of 0.5-1.0 ?m, optical sectioning thicknesses of 1.0-5.0 ?m, and depths of up to 200-300 ?m below the skin surface [5]. This allows the epidermis, papillary dermis, and upper reticular dermis to be seen at resolutions comparable to histology [6-8]. Melanin and melanosomes reflect highly and provide strong contrast from surrounding tissues. Other sources of contrast include keratin, mitochondria, cytoplasmic organelles, nuclear chromatin, and collagen. Cytomorphological aspects, architectural patterns, and cellular borders are assessed to diagnose abnormal lesions [9, 10].
Multiple studies worldwide have shown confocal microscopy to be a sensitive and specific tool for diagnosing skin cancer. A large retrospective study published in 2004 of 152 various lesions in the United States revealed 83% sensitivity and 96% specificity for diagnosing basal cell carcinoma [11]. The first large study for diagnosing melanoma by confocal microscopy was published in 2005. On evaluation of 117 melanocytic lesions in Austria, confocal microscopy achieved 88% sensitivity and 98% specificity for melanoma [10]. Two subsequent followup studies in Austria with additional lesions resulted in 91-94% sensitivity and 98-99% specificity for melanoma [12,13]. Another large study published in 2007 of 351 melanocytic lesions in Italy found 92% sensitivity and 69% specificity for melanoma [9]. In Canada, a prospective study published in 2007 of 125 patients with suspicious pigmented lesions showed 97% sensitivity and 83% specificity for diagnosing melanoma by confocal microscopy [14]. An analysis published in 2007 of 154 melanocytic and nonmelanocytic lesions in Spain found confocal microscopy distinguished malignant from benign lesions with 85% sensitivity and 95% specificity [15]. The largest and newest series published in 2012 with 710 malignant and benign lesions in Australia revealed 100% sensitivity and 89% specificity for basal cell carcinoma, and 88% sensitivity and 71% specificity for melanoma [16]. Many other studies are currently in progress [17].
Although promising, new diagnostic tests present with challenges. One of the drawbacks of confocal microscopy is the high cost of the confocal microscopes, typically running $50,000 to $100,000. Currently available microscopes are also relatively bulky, approximately the size of a small shoebox, and mounted to a mobile computer workstation. However, new instrumentation and innovative methods are simplifying designs and driving down costs as well as size while maintaining imaging quality [18,19]. New, handheld microscopes comparable to the size of a small hair dryer have recently been released. Another drawback of confocal microscopy is resolution limited to the upper layers of the skin. Structures located deeper in the reticular dermis and beyond cannot be examined. Interpretation of images is also subject to interobserver variability. The results are dependent on the expertise and training of the physician interpreting the images. Despite these drawbacks, confocal microscopy provides the ability to rapidly view cellular-level detail within the skin and noninvasively monitor lesions and detect skin cancer.
A skin biopsy or excised tissue sample will remain the gold standard for skin cancer diagnosis. Although not a replacement for biopsy, confocal microscopy can provide an additional layer of information to avoid an unnecessary biopsy. This may benefit patients with a lesion of questionable concern in an anatomically aesthetic site such as the face. Additionally, patients may be spared from undergoing a presurgical biopsy and opt to go straight to surgical excision. Confocal microscopy has already been implemented in a handful of dermatology practices and academic medical centers in the United States. As the technology improves and becomes more widespread, both patients and physicians may have an option aside from an invasive biopsy for detecting skin cancer.
Brian Park is a 4th year medical student at NYU School of Medicine
Faculty peer reviewed by Miriam Pomeranz, MD, Dermatology, NYU Langone Medical Center
Image courtesy of Wikimedia Commons
References
1. Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146(3):283-287. http://www.ncbi.nlm.nih.gov/pubmed/20231499
2. Robinson JK. Sun exposure, sun protection, and vitamin D. JAMA. 2005;294(12):1541-1543. http://jama.jamanetwork.com/article.aspx?articleid=201579
3. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10-29. http://www.ncbi.nlm.nih.gov/pubmed/22237781
4. Nehal KS, Gareau D, Rajadhyaksha M. Skin imaging with reflectance confocal microscopy. Semin Cutan Med Surg. 2008;27(1):37-43.
5. Psaty EL, Halpern AC. Current and emerging technologies in melanoma diagnosis: the state of the art. Clin Dermatol. 2009;27(1):35-45. http://www.ncbi.nlm.nih.gov/pubmed/19095152
6. Marghoob AA, Charles CA, Busam KJ, et al. In vivo confocal scanning laser microscopy of a series of congenital melanocytic nevi suggestive of having developed malignant melanoma. Arch Dermatol. 2005;141(11):1401-1412.
7. Tannous ZS, Mihm MC, Flotte TJ, Gonzalez S. In vivo examination of lentigo maligna and malignant melanoma in situ, lentigo maligna type by near-infrared reflectance confocal microscopy: comparison of in vivo confocal images with histologic sections. J Am Acad Dermatol. 2002;46(2):260-263.
8. Pellacani G, Cesinaro AM, Seidenari S. Reflectance-mode confocal microscopy for the in vivo characterization of pagetoid melanocytosis in melanomas and nevi. J Invest Dermatol. 2005;125(3):532-537.
9. Pellacani G, Guitera P, Longo C, Avramidis M, Seidenari S, Menzies S. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127(12):2759-2765. http://www.ncbi.nlm.nih.gov/pubmed/17657243
10. Gerger A, Koller S, Kern T, et al. Diagnostic applicability of in vivo confocal laser scanning microscopy in melanocytic skin tumors. J Invest Dermatol. 2005;124(3):493-498.
11. Nori S, Rius-Diaz F, Cuevas J, et al. Sensitivity and specificity of reflectance-mode confocal microscopy for in vivo diagnosis of basal cell carcinoma: a multicenter study. J Am Acad Dermatol. 2004;51(6):923-930.
12. Gerger A, Koller S, Weger W, et al. Sensitivity and specificity of confocal laser-scanning microscopy for in vivo diagnosis of malignant skin tumors. Cancer. 2006;107(1):193-200. http://onlinelibrary.wiley.com/doi/10.1002/cncr.21910/full
13. Gerger A, Hofmann-Wellenhof R, Langsenlehner U, et al. In vivo confocal laser scanning microscopy of melanocytic skin tumours: diagnostic applicability using unselected tumour images. Br J Dermatol. 2008;158(2):329-333.
14. Langley RG, Walsh N, Sutherland AE, et al. The diagnostic accuracy of in vivo confocal scanning laser microscopy compared to dermoscopy of benign and malignant melanocytic lesions: a prospective study. Dermatology. 2007;215(4):365-372.
15. Segura S, Puig S, Carrera C, Palou J, Malvehy J. Development of a two-step method for the diagnosis of melanoma by reflectance confocal microscopy. J Am Acad Dermatol. 2009;61(2):216-229.
16. Guitera P, Menzies SW, Longo C, Cesinaro AM, Scolyer RA, Pellacani G. In vivo confocal microscopy for diagnosis of melanoma and basal cell carcinoma using a two-step method: analysis of 710 consecutive clinically equivocal cases. J Invest Dermatol. 2012;132(10):2386-2394.
17. Parsons SK, Chan JA, Yu WW, et al. Agency for Healthcare Research and Quality (US). Noninvasive diagnostic techniques for the detection of skin cancers. http://www.ncbi.nlm.nih.gov/books/NBK82493/. Published September 2012. Accessed September 26, 2012.
18. Dwyer PJ, DiMarzio CA, Zavislan JM, Fox WJ, Rajadhyaksha M. Confocal reflectance theta line scanning microscope for imaging human skin in vivo. Opt Lett. 2006;31(7):942-944. http://www.ncbi.nlm.nih.gov/pubmed/16599219
19. Dwyer PJ, DiMarzio CA, Rajadhyaksha M. Confocal theta line-scanning microscope for imaging human tissues. Appl Opt. 2007;46(10):1843-1851.