Peer Reviewed
Case and Introduction
A 52-year-old right-handed woman with hypertension is brought in by ambulance after her daughter notices a sudden onset of nonsensical speech and trouble walking. On exam, she has an expressive aphasia with right-sided hemiparesis. Her vitals are stable with her blood pressure well-controlled by a home medication. Computed tomography (CT) with angiography reveals signs of early acute ischemic changes in the right middle cerebral artery territory but no large vessel occlusion. She has no metabolic disturbances, an unremarkable complete blood count, and normal coagulation profile. She receives intravenous tissue plasminogen activator with improvement in symptoms and is admitted to the stroke unit. Further work up with 24-hour Holter monitor records a few atrial premature complexes and cardiac ultrasound reveals only a small inter-atrial shunt. At this point, the etiology of her ischemic stroke remains unclear. How should this case be further evaluated?
Despite advances in testing, many ischemic strokes are considered cryptogenic, in which thorough investigation fails to reveal a single probable etiology [1,2]. Less well-understood mechanisms are thought to comprise of cryptogenic strokes including occult arrhythmias, paradoxical emboli, undefined hypercoagulable states, and atherosclerotic disease of the aorta or supra-aortic vasculature [3]. Most cryptogenic stroke are likely embolic, a characteristic supported by neuroimaging observations [4]. To better encompass this understanding and set criteria for minimum diagnostic work-up, the term “embolic stroke of undetermined source” (ESUS) was introduced in 2014. ESUS is determined after significant large vessel atherosclerosis, lacunar or small vessel infarct, major-risk cardioembolic sources, and less common causes like dissection and vasculitis cannot be identified [5]. Complete diagnostic assessment of cryptogenic strokes is less clear and an ongoing field of study. This article seeks to review diagnostic approach to cryptogenic stroke based on available evidence.
Standard Evaluation of Ischemic Stroke
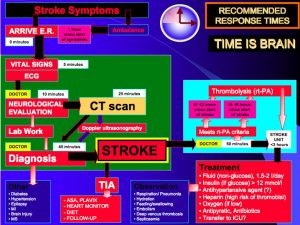
Acute stroke management involves urgent evaluation with rapid brain imaging by noncontrast CT or magnetic resonance imaging (MRI), electrocardiogram (ECG) and troponins as many patients also present with concurrent cardiac ischemia [6], and standard blood tests. In the emergent setting, blood glucose, serum electrolytes, complete blood count, and coagulation profile should be obtained. If an institution offers endovascular recanalization therapies, evaluation of large vessel occlusive disease with noninvasive CT or MR angiography may be helpful in the emergent setting [7].
To evaluate for cardioembolic source of stroke, continuous cardiac monitoring and an echocardiogram are considered part of a basic cardiac evaluation for a cardioembolic source of stroke. While the initial ECG may not detect arrhythmias, all patients with ischemic stroke should have cardiac monitoring for at least 24 hours by continuous telemetry or Holter monitor [7]. The exact timing and length of monitoring remains unspecified by current guidelines. Transesophageal echocardiogram (TEE) offers a better evaluation of common sources of emboli in the left atrium and appendage, inter-atrial septum, and thoracic aorta, but a transthoracic approach may be initially preferred if there is concern for congestive heart failure, coronary artery disease, left ventricular thrombus, or patient contraindication [8,9]. TEE is more sensitive than transthoracic echo (TTE) for cardiac sources of emboli and may identify additional abnormalities in about half of young patients with cryptogenic stroke [10,11]. Therefore, TEE with color Doppler study and agitated saline contrast injection to evaluate for patent foramen ovale (PFO) or atrial septal shunt should be performed if transthoracic sonography is unrevealing.
Advanced Cardiac Monitoring
If standard evaluation cannot identify a probable stroke etiology, the stroke can be considered cryptogenic and warrants advanced evaluation involving prolonged cardiac monitoring, advanced imaging of vessels, and further hematological testing for coagulopathies [9]. In patients with no history of atrial fibrillation (AF) and new implanted pacemaker or defibrillator, asymptomatic atrial tachyarrhythmias were seen in about 10% of patients and associated with a 2.5-fold increased risk in ischemic stroke or systemic embolism, highlighting the value of prolonged monitoring. Of note, the study did not observe any clear temporal relationship between occult paroxysmal AF and stroke, suggesting paroxysmal AF might be a risk factor or marker for other comorbidities that increase risk of stroke rather than act as a primary etiology alone [12]. By combining standard acute inpatient and extended cardiac monitoring in sequence, an estimated occult AF detection yield is as high as 25% of all ischemic stroke patients, uncovering a significant burden of patients who may benefit from anticoagulation [13].
The optimal long-term cardiac monitoring is unclear, though longer durations are associated with greater yield of occult AF detection [14]. Several large studies have demonstrated higher rates of detected occult AF using long-term device monitoring strategies, such as an external loop recorder, as compared to conventional ambulatory screening [15-17]. However, these studies are underpowered to show risk reduction in stroke, transient ischemic attack, or systemic emboli. Further, the minimal duration of paroxysmal AF, or AF burden, needed to increase risk of atrial thrombus formation and ultimately stroke risk is unclear [18].
Practices vary by institution, but many opt for one month of ambulatory external cardiac monitoring after standard evaluation of cryptogenic stroke [9,19]. Further considerations taken to identify patients who have increased likelihood of occult AF after cryptogenic stroke can also aid in monitoring strategies. Patients older than 65 years, embolic stroke patterns on imaging [20], a higher virtual CHA2DS2-VASc score [21], left atrial enlargement [22], characteristic P wave morphology ECG changes [23-25], frequent atrial premature beats on Holter [26], and serum N-terminal pro-brain natriuretic peptide levels [27] are associated with higher occult AF after cryptogenic stroke.
Advanced Vascular Imaging
Standard CT or MR angiography may miss lesions that can be identified by advanced vascular imaging. Conventional invasive angiography remains as the gold standard for imaging intra- and extracranial vasculature [7]. In the acute setting, invasive angiography remains rarely performed given a small risk of stroke and availability of rapid noninvasive modalities, unless endovascular recanalization is already planned. Notably, conventional angiography can visualize smaller arteries, assess collateral flow and perfusion status, determine degree of arterial stenosis, and identify pathologies such as dissection, vasculitis, and vascular malformations [28].
Nonstenotic plaques with vulnerable intraplaque features such as thin fibrous caps and necrotic cores are increasingly recognized as potential sources of microemboli that may evade standard evaluation. [29,30]. Developments in high-resolution MRI allow direct noninvasive visualization of vessel walls to identify vulnerable nonstenotic plaque features, but availability limits this technology [31]. More commonly, transcranial dopplar with microemboli detection monitoring is used to evaluate for asymptomatic microemboli from carotid or cardiac sources that have been implicated in increased stroke and TIA risk [32]. Lastly, cardiac MRI can be considered to identify missed potential sources of embolism on echocardiography, including left ventricular thrombus and complex aortic arch atheromas [33].
Hypercoagulable States
Inherited or acquired hypercoagulopathies are not a well-studied cause for ischemic stroke and thought to only contribute to a small proportion of ischemic stroke. Generally, hypercoagulable work-up for antiphospholipid syndrome and coagulopathies is indicated in select patients, particularly those who are young, with a PFO and possibly at risk of paradoxical embolism, with history of unprovoked venous thromboembolism (VTE), VTE in an usual location, and family history of VTE [10,33]. To contrast, some recent reports suggest there is little benefit to advanced testing for hypercoagulable states in cryptogenic stroke, even in young patients and those with PFOs [34]. Further, identification of hypercoagulable patients may not yield risk reduction in recurrent stroke despite therapy [35]. As a result, many clinicians argue that thrombophilia work-up in stroke is an unjustified cost that can lead to unnecessary anticoagulation [34]. Furthermore, in the setting of an acute stroke, some markers of hypercoagulability can be transiently or falsely elevated, and the tests may need to be repeated. Therefore, it is often more cost effective to perform hypercoagulability testing after other tests have been performed, perhaps in the outpatient rather than the hospital setting.
Conclusions
Despite advances in diagnostic methods, a significant portion of ischemic strokes fails to have a probable cause determined. While standard evaluation of all ischemic strokes is set by American Heart Association/American Stroke Association guidelines, further complete evaluation for cryptogenic strokes is less concise. At minimum, thorough imaging of brain parenchyma and intra- and extracranial vasculature, structural and embolic cardiac work-up, prolonged occult arrhythmia monitoring, and laboratory testing for assessment of immediately reversible causes and sparingly of hypercoagulable states should be obtained. Rare, often genetic, etiologies can also be investigated if suspected. Clinically, while not discussed here, long-term treatment of cryptogenic stroke presents challenges in secondary stroke prevention without specific identifiable targets. Given the recent repackaging of cryptogenic stroke classification into ESUS, many ongoing multicenter trials aim at addressing these uncertainties (NCT02239120, NCT02427126, NCT01938248), with expected primary outcome evaluations to be completed this year.
Dr. Dixon Yang is a 1st year resident at NYU Langone Health
Peer reviewed by Matt Sanger, MD, neurology, NYU Langone Health
Image courtesy of Wikimedia Commons
References
- Sacco RL, Ellenberg JH, Mohr JP, et al. Infarcts of undetermined cause: the NINCDS Stroke Data Bank. Ann Neurol.1989;25(4):382-90. https://www.ncbi.nlm.nih.gov/pubmed/2712533
- Ji R, Schwamm LH, Pervez MA, Singhal AB. Ischemic stroke and transient ischemic attack in young adults: risk factors, diagnostic yield, neuroimaging, and thrombolysis. JAMA Neurol. 2013;70(1):51-7.
- Liberman AL, Prabhakaran S. Cryptogenic stroke: how to definte it? How to treat it? Curr Cardiol Rep. 2013;15(12):423.
- Lamy C, Giannesini C, Zuber M, et al. Clinical and imaging findings in cryptogenic stroke patients with and without patent foramen ovale: the PFO-ASA Study. Atrial Septal Aneurysm. Stroke J Cereb Circ. 2002;33(3):706-11.
- Hart RG, Diener HC, Coutts SB, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13(4):429-38. https://www.ncbi.nlm.nih.gov/pubmed/24646875
- Kerr G, Ray G, Wu O, Stott DJ, Langhome P. Elevated troponin after stroke: a systematic review. Cerebrovasc Dis. 2009;28(3):220-6.
- Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947. https://www.ncbi.nlm.nih.gov/pubmed/23370205
- Saric M, Armour AC, Arnaout MS, et al. Guidelines for the use of echocardiography in the evaluation of a cardiac source of embolism. J Am Soc Echocardiogr. 2016;29(1):1-42.
- Saver JL. Cryptogenic stroke. N Engl J Med. 2016;347(21):2065-74.
- Reynolds HR, Jagen MA, Tunick PA, Kronzon I. Sensitivity of transthoracic versus transesophageal echocardiography for the detection of native valve vegetations in the modern era. J Am Soc Echocardiogr. 2003;16(1):67-70. 21. https://www.ncbi.nlm.nih.gov/pubmed/12514637
- Katsanos AH, Bhole R, Frogoudaki A, et al. The value of transesophageal echocardiography for embolic strokes of undetermined source. Neurology. 2016;87(10):988-95.
- Brambatti M, Connolly SJ, Gold MR, et al. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation. 2014;129(21):2094-9.
- Sposato LA, Cipriano LE, Saposnik G, Vargas ER, Riccio PM, Hachinski V. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol. 2015;14(4):377-87.
- Dussault C, Toeg H, Nathan M, Wang ZJ, Roux JF, Secemsky E. Electrocardiographic monitoring for detecting atrial fibrillation after ischemic stroke or transient ischemic attack: systemic review and meta-analysis. Circ Arrhythm Electrophysiol. 2015;8(2):263-9.
- Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370(26):2467-77.
- Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370(26):2478-86.
- Wachter R, Groschel K, Gelbrich G, et al. Holter-electrocardiogram-monitoring in patients with acute ischaemic stroke (Find-AFRANDOMISED): an open-label randomised controlled trial. Lancet Neurol. 2017;16(4):282-90.
- Boriani G, Glotzer TV, Santini M, et al. Device-detected atrial fibrillation and the risk for stroke: analysis from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation information from implanted devices). Eur Heart J. 2014;35(8):508-16.
- Zhang C, Kasner S. Diagnosis, prognosis, and management of cryptogenic stroke. F1000Res. 2016; pii: F1000 Faculty Rev-168.
- Favilla CG, Ingala E, Jara J, et al. Predictors of finding occult atrial fibrillation after cryptogenic stroke. Stroke. 2015;46(5):1210-5.
- Suzuki S, Sagara K, Otsuka T, et al. Usefulness of frequent supraventricular extrasystoles and a high CHADS2 score to predict first-time appearance of atrial fibrillation. Am J Cardiol. 2013;111(11):1602-7.
- Carrazco C, Golyan D, Kahen M, Black K, Libman RB, Katz JM. Prevalence and risk factors for paroxysmal atrial fibrillation and flutter detection after cryptogenic stroke. J Stroke and Cerberovasc Dis. 2017;S1052-3057(17):30432-9.
- Dilaveris PE, Gialafos JE. P-wave dispersion: a novel predictor of paroxysmal atrial fibrillation. Ann Noninvasive Electrocardiol. 2001;6(2):159-65.
- Guidera SA, Steinberg JS. The signal-averaged P wave duration: a rapid and noninvasive marker of risk of atrial fibrillation. J Am Coll Cardiol. 1993;21(7):1645-51.
- Thijs VN, Brachmann J, Morillo CA, et al. Predictors for atrial fibrillation detection after cryptogenic stroke: results from CRYSTAL AF. Neurology. 2016;86(3):261-9.
- Gladstone DJ, Dorian P, Spring M, et al. Atrial premature beats predict atrial fibrillation in cryptogenic stroke: results from the EMBRACE trial. Stroke. 2015;46(4):936-41.
- Patton KK, Ellinor PT, Heckbert SR, et al. N-terminal pro-B-type natriuretic peptide is a major predictor of the development of atrial fibrillation: the Cardiovascular Health Study. Circulation. 2009;120(18):1768-74.
- Latchaw RE, Alberts MJ, Lev MH, et al. Recommendations for imaging of acute ischemic stroke: a scientific statement from the American Heart Association. 2009;40(11):3646-78.
- Yang D, Iyer S, Gardener H, et al. Cigarette smoking and carotid plaque echodensity in the Northern Manhattan Study. Cerebrovasc Dis. 2015;40(3-4):136-43.
- Madani A, Beletsky V, Tamayo A, Munoz C, Spence JD. High-risk asymptomatic carotid stenosis: ulceration on 3D ultrasound vs TCD microemboli. Neurology. 2011;77(8):744-50.
- Bodle JD, Feldmann E, Swartz RH, Rumboldt Z, Brown T, Turan TN. High resolution magnetic resonance imaging: an emerging tool for evaluating intracranial arterial disease. Stroke. 2013;44(1):287-92.
- Markus HS, King A, Shipley M, et al. Asymptomatic embolisation for prediction of stroke in the Asymptomatic Carotid Emboli Study (ACES): a prospective observational study. Lancet Neurol. 2010;9(7):663-71.
- Fonesca AC, Ferro JM. Cryptogenic stroke. Eur J Neurol. 2015;22(4):618-23.
- Morris JG, Singh S, Fisher M. Testing for inherited thrombophilias in arterial stroke. Stroke. 2010;41(10):2985-90.
- Weber R, Goertler M, Benemann J, et al. Prognosis after cryptogenic cerebral ischemia in patients with coagulopathies. Cerebrovasc Dis. 2009;28(6):611-7.