Peer Reviewed
Recent headlines about increasing rates of metastatic prostate cancer have had many patients asking if they should be tested.1 This article will review the history and role of prostate cancer screening in normal-risk asymptomatic men aged 45-75.
Prostate cancer (PCa) is the most common cancer in men, with 12.6% of men being diagnosed during their lifetimes.2 PCa is responsible for 18.9 deaths per 100,000 men per year, with approximately one-third occurring in patients under age 75.2 What makes PCa particularly challenging is that it is common, rarely symptomatic, rarely metastatic, and is over-diagnosed. The most readily available blood screening test for prostate cancer is prostate-specific antigen (PSA), the use of which in asymptomatic men has been a controversial topic for many years and the subject of numerous epidemiological studies3,4 and large randomized controlled trials.5-7
The United States Preventive Services Task Force (USPSTF) first weighed in on this topic in 2008 when it recommended against screening men aged 75 or older (grade D)8 and in 2012 when it recommended against routine screening for younger men (grade D).9 This second recommendation resulted in a significant decline in PSA usage and an estimated 33,000 prostate cancers going undetected within one year.10
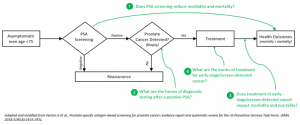
This decision was re-evaluated in 2018 when the Task Force examined several key evidentiary questions, the most salient of which are identified in Figure 1.11 First, the Task Force examined three large randomized-controlled trials (n = 647,906) and found that the use of PSA screening in asymptomatic men provided no mortality benefit (all-cause or prostate-cancer specific) compared to usual care, although a sub-analysis of one study found a significant decrease in metastatic PCa (number needed to screen: 323).5-7,11 Next the Task Force examined the harms of post-screening diagnostic testing, finding 16-28 biopsies were performed per 100 men screened, with 60-76% of all biopsies not identifying cancer.5-7,11 This high rate of biopsies (and unnecessary biopsies) was judged to be significant considering observational studies showing complication rates of 2.0-5.6% per biopsy (including infection, bleeding, or urinary difficulties) and biopsy-associated hospitalizations occurring in 0.5-1.6% of cases.11,12
Should PCa be detected early, the Task Force next examined if early detection and treatment would improve outcomes. It found that three RCTs (n = 2,524) found a statistically improved prostate-cancer-related and all-cause mortality in asymptomatic patients treated with radical prostatectomy and radiation therapy, although several large cohort studies did not support these findings.13-16 These benefits were weighed against the harm of treatment, including a relative risk of 1.82 for developing erectile dysfunction and 2.27 for developing incontinence. More concerningly, large observational studies found that 1.7% of prostatectomy patients had major cardiac or pulmonary complications, 5.3% had major surgical complications, and the perioperative mortality rate was 0.29%.11,17 Assessing these risks and benefits, the USPSTF found the evidence was not clearly compelling, ultimately recommending that physicians and patients engage in shared decision-making to weigh the risks and benefits for individual situations (grade C).18
Unbeknownst to the USPSTF, during the time during which its 2012 recommendation against PSA use in all adults (originally published in 2011)19 was in effect, incidence rates of metastatic PCa among men aged 45 to 74 years in the US increased by 5.3% year-over-year during 2010-2018 after being largely stable for many years prior, increasing the metastatic prostate cancer incidence rate from around 60 per 100,000 to 90 per 100,000.1 This is especially concerning, as metastatic prostate cancer is responsible for the vast majority of PCa mortality and has a 5-year relative survival rate of 32% compared to localized and regional PCa.2 Although not conclusive, it does appear that PSA-associated detection and treatment of PCa likely contributed to lower metastatic PCa rates prior to 2012. Since the USPSTF 2018 recommendation was only recently implemented, and US epidemiology data are only available through 2019, it is difficult to assess if the 2018 shared-decision-making recommendation has had an impact on the metastatic incidence rate, although this analysis is certainly on the horizon.
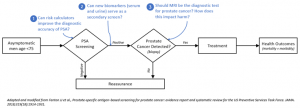
More importantly, much has changed in the world of PCa screening and diagnosis, leading to numerous new questions that must be considered (Figure 2). With the continual development of popular risk-stratifying calculators using large-data approaches, it is possible that PSA testing accuracy could be considerably improved as part of a multifactorial calculator.20,21 Although this was briefly considered by the USPSTF in 2018, the lack of sufficient evidence for relatively untested risk calculators prevented their use from being recommended.11 Continued research and development of more accurate calculators (especially those that predict high-stage cancer) is an area of continued research that could potentially bring PSA back into more regular use.22 Another area of research has been the identification of novel serum and urine biomarkers that could potentially be used as a second screen after PSA.23 Even more intriguingly, there are some biomarkers that have diagnostic value specifically for detecting aggressive PCa, which could help refine risk stratification.24,25
One area that has changed considerably since the USPSTF’s 2018 guidelines is the principal diagnostic test for PCa. A guideline published in 2020 by the European Association of Urology and other groups recommends multiparametric magnetic resonance imaging (MRI) as first-line testing in order to avoid unnecessary biopsies.26 This was in response to a 2019 Cochrane meta-analysis that found that MRI had improved diagnostic accuracy compared to systematic biopsy.27 Although MRI exams carry a significant financial cost, the ability to use MRI exams to reduce unnecessary biopsies would significantly reduce the physical harm of a false-positive PSA. However, adding steps and costs to the process of diagnosing PCa could also increase the psychological burden of PSA screening.11
In conclusion, the USPSTF’s 2018 recommendation for a shared-decision-making approach to PSA screening must now be re-evaluated considering increasing metastatic prostate cancer rates. As prostate cancer detection approaches continue to be refined using large data for risk-stratification, employing novel biomarkers, and leveraging MRI scanning to avoid biopsies, it is possible that routine PSA screening may provide a previously unseen mortality benefit with less risk than before.
Figure 1: Diagnostic screening pathway for prostate cancer using PSA, with annotated key questions considered by the USPSTF.
Figure 2: Diagnostic screening pathway for prostate cancer using PSA, with annotated key questions for the future considering new evidence and technology.
By Ian Jaffe is a 3rd year medical student at NYU Grossman School of Medicine
Reviewed by Michael Tanner, MD, associate editor, Clinical Correlations
Image courtesy of Wikimedia Commons, source: [[File:Prostate – Gray1153.png|Prostate_-_Gray1153]]
References
- Desai MM, Cacciamani GE, Gill K, et al. Trends in incidence of metastatic prostate cancer in the US. JAMA Netw Open. 2022;5(3):e222246.
- SEER Cancer Stat Facts: Prostate Cancer. National Cancer Institute; 2022. https://seer.cancer.gov/statfacts/html/prost.html. Accessed June 18, 2022.
- Grönberg H. Prostate cancer epidemiology. Lancet. 2003;361(9360):859-864.
- Brawley OW. Prostate cancer epidemiology in the United States. World J Urol. 2012;30(2):195-200.
- Martin RM, Donovan JL, Turner EL, et al. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA. 2018;319(9):883-895.
- Andriole GL, Crawford ED, Grubb III RL, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360(13):1310-1319.
- Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320-1328.
- U. S. Preventive Services Task Force. Screening for colorectal cancer: U. S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(9):627-637.
- Moyer VA, U. S. Preventive Services Task Force. Screening for prostate cancer: U. S. Preventive Services Task Force recommendation statement. Ann Intern Med.. 2012;157(2):120-134.
- Jemal A, Fedewa SA, Ma J, et al. Prostate cancer incidence and PSA testing patterns in relation to USPSTF screening recommendations. JAMA. 2015;314(19):2054-2061.
- Fenton JJ, Weyrich MS, Durbin S, Liu Y, Bang H, Melnikow J. Prostate-specific antigen–based screening for prostate cancer: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319(18):1914-1931.
- Walter LC, Fung KZ, Kirby KA, et al. Five-year downstream outcomes following prostate-specific antigen screening in older men. JAMA Intern Med. 2013;173(10):866-873.
- Wilt TJ, Jones KM, Barry MJ, et al. Follow-up of prostatectomy versus observation for early prostate cancer. New Engl J Med. 2017;377(2):132-142.
- Hamdy FC, Donovan JL, Lane J, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375(15):1415-1424.
- Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. New Engl J Med. 2014;370(10):932-942.
- Barocas DA, Alvarez J, Resnick MJ, et al. Association between radiation therapy, surgery, or observation for localized prostate cancer and patient-reported outcomes after 3 years. JAMA. 2017;317(11):1126-1140.
- Rabbani F, Yunis LH, Pinochet R, et al. Comprehensive standardized report of complications of retropubic and laparoscopic radical prostatectomy. Eur Urol. 2010;57(3):371-386.
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319(18):1901-1913.
- Hoffman RM. Striking the right balance with prostate cancer screening. JAMA Netw Open. 2022;5(3):e222174-e222174.
- Mitka M. Data-based risk calculators becoming more sophisticated—and more popular. JAMA. 2009;302(7):730-731.
- Osses DF, Roobol MJ, Schoots IG. Prediction medicine: biomarkers, risk calculators and magnetic resonance imaging as risk stratification tools in prostate cancer diagnosis. Int J Mol Sci. 2019;20(7):1637.
- Huynh-Le M-P, Fan CC, Karunamuni R, et al. A genetic risk score to personalize prostate cancer screening, applied to population data. Cancer Epidemiol Biomarkers Prev. 2020;29(9):1731-1738.
- Duffy MJ. Biomarkers for prostate cancer: prostate-specific antigen and beyond. Clin Chem Lab Med. 2020;58(3):326-339.
- Fujita K, Nonomura N. Urinary biomarkers of prostate cancer. Int J Urol. 2018;25(9):770-779.
- Filella X, Fernández-Galan E, Bonifacio RF, Foj L. Emerging biomarkers in the diagnosis of prostate cancer. Pharmacogenomics Pers Med. 2018;11:83-94.
- Mottet N, van den Bergh RC, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer—2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79(2):243-262.
- Drost FH, Osses D, Nieboer D, et al. Prostate magnetic resonance imaging, with or without magnetic resonance imaging-targeted biopsy, and systematic biopsy for detecting prostate cancer: a Cochrane systematic review and meta-analysis. Eur Urol. 2020;77(1):78-94.