Commentary By Susan Morey PharmD, Pharmacy Practice Resident
Approximately 3% of patients who use sulfonamide antibiotics develop an allergic reaction, with the most common being the development of a maculopapular rash. (1, 2, 3) Sulfonamides are chemical compounds which contain a SO2NH2 moiety and can be divided into 3 groups based on their structure. (1, 2)Â The first group, the sulfonylarylamines includes the sulfonamide antibiotics. The second group, the nonsulfonylarylamines, includes carbonic anhydrase inhibitors (CAI), cyclooxygenase 2 (COX-2) inhibitors, loop diuretics, thiazides, and sulfonylureas and the protease inhibitors amprenavir and fosamprenavir. (1, 2) The third group is labeled sulfonamide-moiety containing drugs and includes 5-HT antagonists and other agents including zonisamide (1, 2).Â
Two types of allergic reactions have been described in relation to these compounds. One being an IgE mediated maculopapular or urticarial rash developing 1-3 days after medication initiation and the other being a hypersensitivity reaction. The hypersensitivity reaction, consisting of fever and non-urticarial rash, usually develops 7-14 days after medication initiation. (3)Â
 Cross-reactivity between the sulfonamide antibiotics and nonantibiotics has been poorly documented over the past 50 years and has remained a topic of debate. Most of the data consists of case reports suggesting that patients who have an allergy to sulfonylarylamines have an increased risk of allergy to a medication in another sulfonamide group. (1) Upon critical review of many of the cases, by Johnson et al, it was found that other factors may have accounted for the reported cross-reactivity, thus rendering the data inconclusive. (3)  Johnson et al evaluated literature associated with several sulfonamide drug classes including carbonic anhydrase inhibitors (CAI), sulfonylureas, loop diuretics, thiazide diuretics, 5-HT agnonists, and COX-2 inhibitors. Sulfonylureas have also been evaluated due to their structure and reports of cross-reactivity. It was noted in a survey of 82 clinic patients on a sulfonylurea, 34 had used a sulfonylarylamines previously. Seven of these patients reported allergy to sulfonylarylamines. Of the patients taking a sulfonylurea, 2 reported a rash. Only one of the two patients had a previous history of sulfonyarylamine allergy. This is an observational study and conclusions must be drawn cautiously however the authors of the survey note it may not be necessary to preclude use of a sulfonylurea in a patient with sulfonamide allergy.   When reviewing thiazide diuretics, Johnson et al, discussed case reports involving indapamide. In a series of 13 patients with skin rash associated with indapamide administration were then administered chlorthalidone, hydrochlorothiazide, furosemide, epitizide or clopamide. There was no recurrence of rash in any of the 13 patients. Several studies have reported on the use of COX-2 inhibitors in patients with a sulfonamide allergy. Three meta-analyses, conducted by Patterson et al, looked at data from 14 randomized controlled trials. Patients with reported sulfonylarylamine allergies and non-sulfonylarlyamine allergies who received celecoxib, a placebo or an active comparator were included. In this meta-analysis there was no statistically significant difference between the 3 groups in terms of potential allergic events. Additionally patients taking celecoxib with and without other nonsulfonylarylamines were compared. In the last of the three meta-analyses the incidence of allergic reactions with celecoxib was compared to the active comparators. No statistically significant findings were noted in these meta-analyses. Based on these findings the authors conclude the risk of cross-reactivity between sulfonamides and celecoxib is low. Based on this review Johnson et al make the recommendation that patients with a sulfonylarylamine allergy can be administered medications from the other two groups with proper monitoring if it is not possible to use an unrelated product. (3)Â
A retrospective cohort study by Strom et al concluded that a history of sulfonamide antibiotic allergy leads to increased risk of allergy with sulfonamide nonantibiotics. However, they also note, that patients with sulfonamide allergies are also at risk for penicillin allergies, suggesting the risk is not only with sulfonamides. The United Kingdom (UK) General Practice Research Database was used to identify patients who were prescribed a sulfonamide antibiotic and then subsequently prescribed a nonantibiotic sulfonamide within 60 days. Patients with an allergic reaction within 30 days of administration of the sulfonamide antibiotic were identified and compared to those from the source group who did not have a reaction. The risk of subsequent allergies to penicillin was also evaluated. The investigators used a narrow definition of allergy (urticaria, anaphylactic shock, erythema multiforme, and drug allergy) and a broad definition of allergy (included asthma, eczema, and unspecified adverse events to a drug). The odds ratio (OR) for association between an allergic reaction after a sulfonamide nonantibiotic and history of allergy to sulfonamide antibiotic, compared to no history of allergy was 6.6 (95% CI, 5.2-8.4) using the broad definition. Using the narrow definition the OR was 13.2 (95% CI 1.7-99.9). The adjusted OR after controlling for sex, age, presence or absence of preexisting asthma, prior asthma medications or corticosteroids was 2.8 (95% CI 2.1-3.7). The OR for patients who had an allergic reaction to penicillin after a reaction for a sulfonamide antibiotic compared to those with no reaction was 7.8 (95% CI 7.1-8.5) with an adjusted OR of 3.9 (95% CI 3.5-4.3). Based on these findings it is not clear that an allergy to a sulfonamide antibiotic places a patient at risk specifically for an allergy to a sulfonamide nonantibiotic but rather places the patient at an increased risk of developing an allergy to other medications in general.  The investigators concluded that prescribers be aware that patients with history of allergic reactions may be at increased risk for all allergic events related to medications. (2)Â
A large number of sulfonamide products contain FDA approved labeling indicating a precaution or contraindication in patients with a “sulfa†allergy. Controversy still exists regarding the validity of data to suggest cross-reactivity between sulfonamide antibiotics and nonantibiotics. Prescribers should still be aware of the cross-reactivity risk and monitor patients carefully when starting a sulfonamide product in a patient with history of allergy.
References:
1. Boehringer SK. Cross-Reactivity of Sulfonamide Drugs. Pharmacists Letter. Nov. 2005;21:1-8.
2. Strom BL, Schinnar R, Apter AJ, Margolis DJ, Lautenbach E, Hennessy S, Bilker WB, Pettitt D. Absence of Cross-Reactivity between Sulfonamide Antibiotics and Sulfonamide Nonantibiotics. New England Journal of Medicine. 2003; 349(17):1628-1635.
3. Johnson KK, Green DL, Rife JP, Limon L. Sulfonamide Cross-Reactivity: Fact or Fiction? Annals of pharmacotherapy. 2005;39:290-301.

7 comments on “Clinical Pharmacy Corner: Sulfonamide Allergy and Cross-Reactivity”
I am an endocrinologist who has used sulfonylureas for thirty years without regard to prior “sulfa” allergy. I have never recognized any allery to sulfonylureaas.
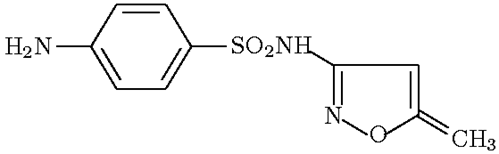
The structural formula depicted of sulfamethoxazole is incorrect. The methyl group connected to the heterocyclic isoxazol ring is connected by a single bond and not a double bond. Remember your organic chemistry-carbon has a “valence” of four and not five.
True, true and the Nobel prize for chemistry goes to…. tam tam tam…. Charles Maltz… Now let’s get back to business. The main problem mentioned here is a rash that may appear in some cases, but a few, just a few… no one can predict that: http://www.interventions.mobi/18/alcohol-rehabs-are-the-only-solace-for-people-with-alcoholism/
I have certainly seen a horrendous reaction to sulfonamides and a subsequent reaction to COX-2 inhibitors. “Rash” does not do justice to the reaction. Liver enzymes were elevated, fatigue was extreme, there were at least two rashes AND urticaria, accompanied by a precipitous drop in the white cell count. Just because you haven’t seen it, doesn’t mean it doesn’t occur.
Correct structure of sulfamethoxazole can be seen and saved as jpg file at:
http://chem.sis.nlm.nih.gov/chemidplus/ProxyServlet?objectHandle=DBMaint&actionHandle=default&nextPage=jsp/chemidheavy/ResultScreen.jsp&ROW_NUM=0&TXTSUPERLISTID=0000723466
I think the bottom line is yes it can occur but it is rare. We must remain informed but know that may more life-years will be saved with these medications than will be lost from adverse effects.
I think the nature of the reaction is important. A reaction like anaphylaxis, urticaria and shortness of breath with Sulfur antibiotic is more worrisome. Mild reactions are not an absolute contraindications to topical use of agents such as dorzolamide or brinzolamide which do not carry risk of mortality.
Comments are closed.