 By Todd Cutler
By Todd Cutler
Faculty Peer Reviewed
A 31-year-old woman presents to the clinic with chronic fatigue. She was diagnosed with iron  deficiency anemia when she was 25 years …

 By Todd Cutler
By Todd Cutler
Faculty Peer Reviewed
A 31-year-old woman presents to the clinic with chronic fatigue. She was diagnosed with iron  deficiency anemia when she was 25 years …
 By Emily Slater
By Emily Slater
Faculty Peer Reviewed
Mr. R is a 46-year-old man with a past medical history of polycythemia vera on hydroxyurea and chronic hepatitis B and C who presented with acutely worsening left …
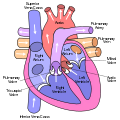 By Brad Pfeffer, MD
By Brad Pfeffer, MD
Faculty Peer Reviewed
Case: A 75- year-old non-smoking male with a history of type II diabetes, hypertension and hyperlipidemia comes to clinic with several months …
Ramin Shayegan Hastings MD, Jonathan Willner MD, and Steven Sedlis MD
Introduction to Cases:
During the past several weeks, we have posted a series of cases addressing the appropriate treatment for patients with stable coronary artery disease. We …
 By Radhika Sundararajan, M.D., Ph.D
By Radhika Sundararajan, M.D., Ph.D
Faculty Peer Reviewed
A healthy 18 year-old female presents to Urgent Care after slipping and falling this morning in the bathroom and hitting her head on the tile floor. She …
Vivian Hayashi MD and Robert Smith MD, Mystery Quiz Section Editors
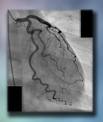
The answer to the mystery quiz is pleural effusion that is loculated in both the horizontal and right oblique fissures. Pleural effusion is seen as blunting of the right costophrenic angle and tracking of fluid laterally (Image 3, …
Vivian Hayashi MD and Robert Smith MD, Mystery Quiz Section Editors
The patient is a 61 year old man presenting with one month of worsening shortness of breath. The patient has a history of alcoholism and was diagnosed with atrial fibrillation during a hospital admission for detox three years earlier. Warfarin …

Introduction to Cases:
During the upcoming weeks, we will post a series of cases addressing the appropriate treatment for patients with stable coronary artery disease. We will be focus on indications for revascularization in stable …