 By Ilina Datkhaeva
By Ilina Datkhaeva
Faculty Peer Reviewed
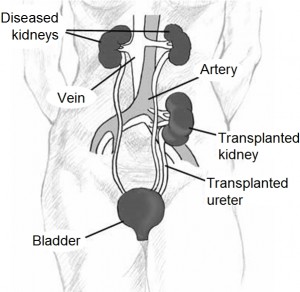
We give hope to patients with advanced kidney disease that a transplant will save them from their Monday, Wednesday, Friday trips to the dialysis unit. But how …

 By Ilina Datkhaeva
By Ilina Datkhaeva
Faculty Peer Reviewed
We give hope to patients with advanced kidney disease that a transplant will save them from their Monday, Wednesday, Friday trips to the dialysis unit. But how …
 By Jon-Emile S Kenny, MD
By Jon-Emile S Kenny, MD
Faculty Peer Reviwed
A 62- year-old man with a history of hypertension, diastolic dysfunction and chronic kidney disease is admitted 4 days after beginning outpatient treatment of community acquired …
 By Jennifer Mulliken
By Jennifer Mulliken
Faculty Peer Reviewed
Case 1:
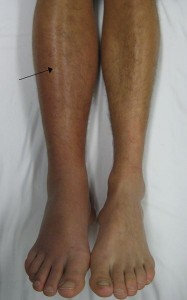
A 30-year-old African-American male with a history of bilateral pulmonary emboli presents with a 1-week history of bilateral lower extremity edema. Blood pressure is …
 Please enjoy this post from the archives first posted on October 21, 2009.
Please enjoy this post from the archives first posted on October 21, 2009.
By Ivan Saraiva MD
Case: A 68-year-old man, with a history of stable angina and end-stage renal disease treated by hemodialysis for the past three years, presents to …
 By Mario V Fusaro, MD
By Mario V Fusaro, MD
Faculty Peer Reviewed
There are certain laws in the universe that are just not meant to be broken. One is gravity. Another one is relativity. The third, don’t give contrast to people with bad kidneys.  Perhaps …
 By Jessica Leifer
By Jessica Leifer
Faculty Peer Reviewed
As a new third year medical student excited to finally be seeing my own patients and still looking for my style as an …
 By Todd Cutler, MD
By Todd Cutler, MD
Faculty Peer Reviewed
A 62-year-old male is hospitalized with an acute congestive heart failure exacerbation. On hospital day three, the patient’s symptoms have significantly …
 By Jon-Emile Kenny, MD
By Jon-Emile Kenny, MD
Faculty Peer Reviewed
A 37-year-old man, with no past medical history and taking finasteride for male pattern baldness, is admitted to Medicine with profound lower …