 By Benjamin Lok
By Benjamin Lok
Faculty Peer Reviewed
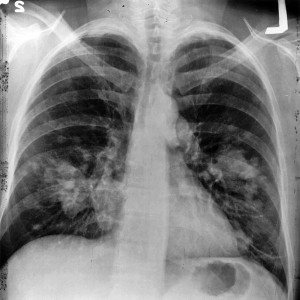
Lung cancer is the most common cause of cancer deaths globally [1] and responsible for an estimated 221,120 new cases and 156,940 deaths in …

 By Benjamin Lok
By Benjamin Lok
Faculty Peer Reviewed
Lung cancer is the most common cause of cancer deaths globally [1] and responsible for an estimated 221,120 new cases and 156,940 deaths in …
 By Demetrios Tzimas, MD
By Demetrios Tzimas, MD
Faculty Peer Reviewed
You are about to discharge a 75-year-old female with hyperlipidemia, hypertension, peripheral vascular disease, who was admitted to the hospital for an ischemic stroke. Being an astute …
 By Aviva Regev
By Aviva Regev
Faculty Peer Reviewed
Introduction
Few people these days are unaware of the “obesity epidemic,†with its inception here in the United States and its steady, insidious spread around the globe. …
By Joseph Larese
Faculty Peer Reviewed
Hemoglobin A1C (HbA1c) is an invaluable tool for monitoring long-term glycemic control in diabetic patients. However, many clinicians managing diabetics have encountered the problem of HbA1c values that do not agree with fingerstick glucose logs. Before suspecting an improperly calibrated glucometer or poor patient record keeping, …
 Please enjoy this post from the archives on July 16, 2009
Please enjoy this post from the archives on July 16, 2009
By Chau Che MD
Faculty Peer Reviewed
You’ll have increased energy, radiant skin, reduced joint pain, improved asthma symptoms, and best of all…you will lose weight. These are some of the …
 By Kaley Myer, Class of 2012
By Kaley Myer, Class of 2012
Faculty Peer Reviewed
As a female, I like the idea of males taking hormonal contraceptives. In a semi-sadistic way, I relish the idea of a man …
 By Robert Gianotti, MD
By Robert Gianotti, MD
Faculty Peer Reviewed
Case: Mr. T is a 32-year-old male being treated by the oncology service for Acute Myelogenous Leukemia. You are the night float intern covering overnight …
 Please enjoy this post from the Archives, first published on July 30, 2009
Please enjoy this post from the Archives, first published on July 30, 2009
By Sam Rougas MD
Faculty Peer Reviewed
It seems that every week a new article in a major newspaper is …