 By Iulia Giuroiu, MD
By Iulia Giuroiu, MD
Peer Reviewed
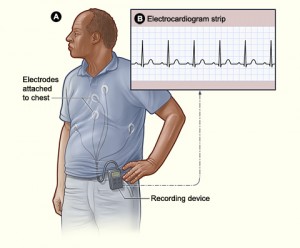
A 70-year-old woman with hypertension, early dementia, and non-specific chest pain of unclear etiology presents with recurrent left-sided chest pain. Unfortunately, she is a poor historian; it appears that her …

 By Iulia Giuroiu, MD
By Iulia Giuroiu, MD
Peer Reviewed
A 70-year-old woman with hypertension, early dementia, and non-specific chest pain of unclear etiology presents with recurrent left-sided chest pain. Unfortunately, she is a poor historian; it appears that her …
 By Kerrilynn Carney, MD
By Kerrilynn Carney, MD
Peer Reviewed
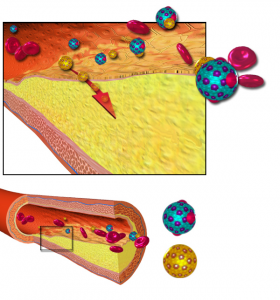
Coronary heart disease remains the leading cause of death globally despite the use of statin therapy. Although major statin studies suggest an average 31% reduction in relative risk of …
 By Steven Bolger
By Steven Bolger
Peer Reviewed
Omega-3 fatty acids were first identified as a potential agent to prevent and treat cardiovascular disease through several epidemiologic studies of the Greenlandic Inuit in the 1970s suggesting that high …
 Cindy Fei, MD
Cindy Fei, MD
Peer Reviewed
A 63-year-old woman with hypertension presents to your clinic for routine follow-up. She came across an online article regarding C-reactive protein and its purported link to heart disease, and she asks you whether she should be tested for …
 By Shannon Chiu, MD
By Shannon Chiu, MD
Peer Reviewed
The annual incidence of infective endocarditis (IE) is estimated to be 3 to 9 cases per 100,000 persons in developed countries [1-2]. Neurologic complications are the most severe and …
 By Anjali Varma Desai, MD
By Anjali Varma Desai, MD
Peer Reviewed
Mr. Q is a 55-year-old male smoker who presents with recurrent chest pain in the mornings over the past several months. The patient reports being awakened from sleep at approximately 5:00 a.m. each morning …
 By Matthew Shou Lun Lee, MD
By Matthew Shou Lun Lee, MD
Peer Reviewed
Clinical Questions
-How common are elevated cardiac enzymes during Wellens’ syndrome?
-Can the EKG changes in Wellens’ syndrome be found with other causes?
Background
This post represents a follow-up …
 By Joshua Michael Lader, MD
By Joshua Michael Lader, MD
Peer Reviewed
As physicians, we are frequently asked to weigh-in on dinnertime discussions about topics that, despite their relevance to everyday life, were never formally addressed in our medical …