 By Gregory Katz, MD
By Gregory Katz, MD
Faculty Peer Reviewed
Everyday in clinic, we tell our patients to choose foods low in saturated fat. Because these foods raise plasma cholesterol, the thinking goes, they cause …

 By Gregory Katz, MD
By Gregory Katz, MD
Faculty Peer Reviewed
Everyday in clinic, we tell our patients to choose foods low in saturated fat. Because these foods raise plasma cholesterol, the thinking goes, they cause …
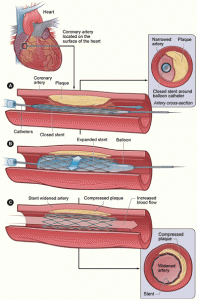 By Anish Vani
By Anish Vani
Faculty Peer Reviewed
According to the 2010 Heart Disease and Stroke Statistics update of the American Heart Association, there are 17.6 million Americans living with coronary heart disease (CHD) [1]. …
Please enjoy this post from the Archives dated September 29, 2010
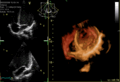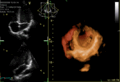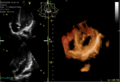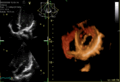 By Lara Dunn, MD
By Lara Dunn, MD
Faculty Peer Reviewed
In 1958, EC Heyde published 10 cases of aortic stenosis (AS) and arteriovenous malformations (AVMs) of the gastrointestinal tract in the New England …
 By Sunny N. Shah, MD
By Sunny N. Shah, MD
Faculty Peer Reviewed
Background:
Atrial fibrillation (AF) is the most common cardiac arrhythmia and its prevalence increases with age. In fact, the lifetime incidence of AF is approximately …
 By Christopher Graffeo
By Christopher Graffeo
Faculty Peer Reviewed
In the age of prevention, primary care is more empowered than ever to educate patients on reducing their risk for common chronic diseases by promoting behavior …
 By Tyler R. McClintock
By Tyler R. McClintock
Faculty Peer Reviewed
“Red Meat Kills.†“Red Meat a Ticket to Early Grave.†“A Hot Dog a Day Raises Risk of Dying.†Such were the headlines circulating in …
 By Lauren Foster
By Lauren Foster
Faculty Peer Reviewed
Hypertension is a pervasive chronic disease affecting approximately 65 million adults in the United States, and a significant cause of morbidity and mortality [1]. …
 Please enjoy this post from the archives dated August 26, 2010
Please enjoy this post from the archives dated August 26, 2010
By Aditya Mattoo, MD
Faculty Peer Reviewed
Not too long ago, a patient came to my clinic and said (I’m paraphrasing of course), “I never cared for alcohol, doctor, so I …