 By Jeffrey Krutoy, DDS
By Jeffrey Krutoy, DDS
Faculty Peer Reviewed
Bacterial infective endocarditis is a potentially devastating disease, and while it may be an easy tradition to blame the dentist, recent research and new guidelines from …

 By Jeffrey Krutoy, DDS
By Jeffrey Krutoy, DDS
Faculty Peer Reviewed
Bacterial infective endocarditis is a potentially devastating disease, and while it may be an easy tradition to blame the dentist, recent research and new guidelines from …
 By Nicole Sunseri
By Nicole Sunseri
Faculty Peer Reviewed
In Africa, there lurks a stealthy and powerful beast. Is it a lion, a black mamba, or a crocodile? No, it is the Anopheles mosquito. Although less than …
 By Kevin Burns
By Kevin Burns
Faculty Peer Reviewed
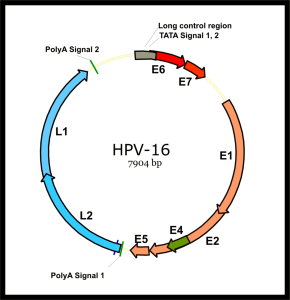
On December 22, 2010, the US Food and Drug Administration (FDA) approved the quadrivalent human papillomavirus (HPV) vaccine (Gardasil; Merck, Whitehouse Station, New Jersey) for prevention …
 By Santosh Vardhana, MD/PhD
By Santosh Vardhana, MD/PhD
Faculty Peer Reviewed
Ms. T is a 32- year-old woman with no past medical history who presents with a three month history of productive cough, shortness of breath, …
 By Mitchell Kim
By Mitchell Kim
Faculty Peer Reviewed
Mycobacterium tuberculosis, an acid-fast bacillus, is the causative agent of tuberculosis (TB), an infection that causes significant morbidity and mortality worldwide. A highly contagious infection, TB …
 Please enjoy this post from the Archives, first published on July 30, 2009
Please enjoy this post from the Archives, first published on July 30, 2009
By Sam Rougas MD
Faculty Peer Reviewed
It seems that every week a new article in a major newspaper is …
 By Todd Cutler, MD
By Todd Cutler, MD
Faculty Peer Reviewed
An 82-year-old man is admitted to the intensive care unit with fevers, hypoxic respiratory failure and hypotension. He is intubated and resuscitated with intravenous fluids. A central venous catheter is …
 By Jessie Yu
By Jessie Yu
Faculty Peer Reviewed
A healthy 21-year-old female college student presents to clinic after one day of dysuria and increased frequency. You diagnose her with a recurrent urinary tract infection (UTI), and as you hand her a prescription …