 By Matt Johnson, MD
By Matt Johnson, MD
Faculty Peer Reviewed
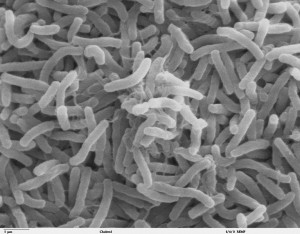
In the fall of 2010, after Haiti was razed by a magnitude 7.2 earthquake that left over 316,000 people dead, cholera was injected into the tumult to add to the growing list of Haiti’s …

 By Matt Johnson, MD
By Matt Johnson, MD
Faculty Peer Reviewed
In the fall of 2010, after Haiti was razed by a magnitude 7.2 earthquake that left over 316,000 people dead, cholera was injected into the tumult to add to the growing list of Haiti’s …
 By Benjamin Bearnot
By Benjamin Bearnot
Faculty Peer ReviewedÂ
Since the discovery of zidovudine (AZT) in the mid-1980s, advances in antiretroviral (ARV) therapy for patients with chronic human immunodeficiency virus (HIV) infection have, until recently, outpaced concomitant …
 By: Aviva Regev
By: Aviva Regev
Mr. S is a 68-year old man with longstanding COPD and a 40-pack-year smoking history. He presents to clinic with three days of increasing shortness of breath, and complains that he has been …
 By Andrea Mignatti , MD
By Andrea Mignatti , MD
Faculty Peer Reviewed
Among all the new medical therapies, this one will probably not be the most elegant or refined that you will read about. But it …
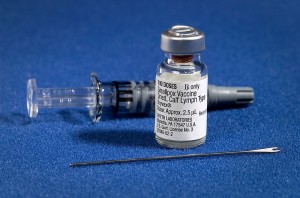 By Michael Cohen
By Michael Cohen
Faculty Peer Reviewed
The varicella-zoster virus (VZV) is well known to the majority of the population. In children, it strikes as varicella (chickenpox), characterized by pruritic, vesicular lesions in different stages of development dispersed over the body. …
 By Ramoncito David
By Ramoncito David
Faculty Peer Reviewed
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death in the world.[1] Â The prevalence of this fatal disease greatly varies among different nations, due to the fact that almost 80% of cases are …
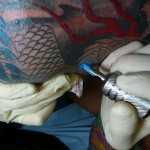 By Farzon A. Nahvi
By Farzon A. Nahvi
Faculty Peer Reviewed
 Once thought to be exclusively the domain of gang members, prisoners, and those in the military, tattoos are now increasingly popular with …
 By Bradley Ching, Class of 2011
By Bradley Ching, Class of 2011
Faculty Peer Reviewed
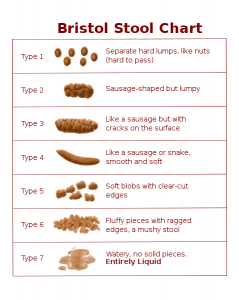
 What do every road trip, football game halftime, and trans-continental plane flight have in common? Usually a disgusting toilet paired …