 By Robert Joseph Fakheri, MD
By Robert Joseph Fakheri, MD
Faculty Peer Reviewed
A 55 year-old male is recently diagnosed with systemic sarcoidosis. The patient is started on prednisone 40mg with the plan to decrease the dose after …

 By Robert Joseph Fakheri, MD
By Robert Joseph Fakheri, MD
Faculty Peer Reviewed
A 55 year-old male is recently diagnosed with systemic sarcoidosis. The patient is started on prednisone 40mg with the plan to decrease the dose after …
 By Natalie Smith, MD
By Natalie Smith, MD
Faculty Peer Reviewed
A few weeks ago I received an email from a friend I grew up with containing a link to an article she had seen online and a …
 By Han Na Kim
By Han Na Kim
Faculty Peer Reviewed
Case:
The patient is a 50-year-old woman with history of steroid-dependent, severe, persistent asthma since childhood and coronary artery disease who presented with dyspnea and …
 By Brian D. Clark
By Brian D. Clark
Faculty Peer Reviewed
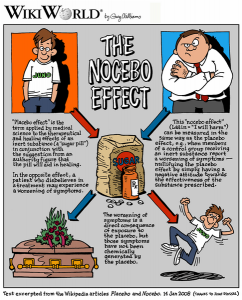
The ability to critically assess the validity of a clinical trial is one of many important skills that a physician strives to develop. This …
 By Santosh Vardhana, MD/PhD
By Santosh Vardhana, MD/PhD
Faculty Peer Reviewed
Ms. T is a 32- year-old woman with no past medical history who presents with a three month history of productive cough, shortness of breath, …
 By Ishmeal Bradley, MD
By Ishmeal Bradley, MD
Faculty Peer ReviewedÂ
The goal of public health is to prevent or minimize disease and injury on a population level. How to achieve this end has changed …
 By Maryann Kwa, MD
By Maryann Kwa, MD
Faculty Peer Reviewed
131 million: The number of times the most popular medication in the United States was prescribed in 2010.
Recently, IMS Health, a company that monitors annual sales for pharmaceutical and healthcare industries, published a …
 By Santosh Vardhana, MD
By Santosh Vardhana, MD
A 36-year-old obese male with hypertension and hyperlipidemia presents to the ER with new knee pain, swelling, and erythema. Joint aspiration reveals negatively birefringent crystals. He is started on oral prednisone.
A 26-year-old woman with lupus presents …