 By Kristina Cieslak, MD
By Kristina Cieslak, MD
Peer Reviewed
Food allergies affect approximately 8% of children and 5% of adults, with a steadily increasing prevalence [1]. Risk factors for the development of food allergy are numerous and …

 By Kristina Cieslak, MD
By Kristina Cieslak, MD
Peer Reviewed
Food allergies affect approximately 8% of children and 5% of adults, with a steadily increasing prevalence [1]. Risk factors for the development of food allergy are numerous and …
 Zachary Elkin
Zachary Elkin
Faculty Peer Reviewed
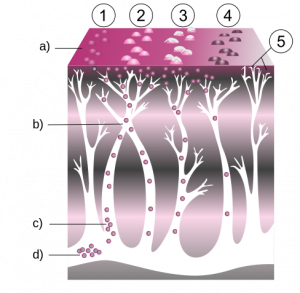
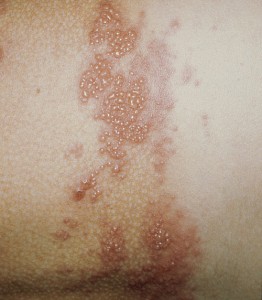
There are more than a million cases of herpes zoster (HZ) in the US annually [1-3]. The incidence of HZ, or shingles, has been rising in the US since the …
 By Jenny Gartshteyn
By Jenny Gartshteyn
Faculty Peer Reviewed
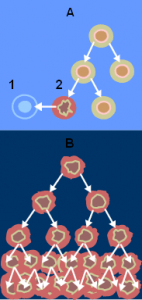
Since the start of vaccination – we’ve eradicated smallpox and polio, saved college kids from meningitis, averted flu epidemics, and decreased the incidence of HPV-related cervical cancer … but can we teach our immune systems to actively …
 By Zachary Elkin
By Zachary Elkin
Faculty Peer Reviewed
There are more than a million cases of herpes zoster (HZ) in the US annually [1-3]. The incidence of HZ, or shingles, has been rising in the US since …
 By Kevin Burns
By Kevin Burns
Faculty Peer Reviewed
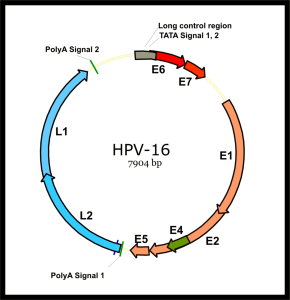
On December 22, 2010, the US Food and Drug Administration (FDA) approved the quadrivalent human papillomavirus (HPV) vaccine (Gardasil; Merck, Whitehouse Station, New Jersey) for prevention of anal cancer …
 By Mitchell Kim
By Mitchell Kim
Faculty Peer Reviewed
Mycobacterium tuberculosis, an acid-fast bacillus, is the causative agent of tuberculosis (TB), an infection that causes significant morbidity and mortality worldwide. A highly contagious infection, TB is spread by …
 By Michael Cohen
By Michael Cohen
Faculty Peer Reviewed
The varicella-zoster virus (VZV) is well known to the majority of the population. In children, it strikes as varicella (chickenpox), characterized by pruritic, vesicular lesions in different stages of development dispersed over the body. A self-resolving …
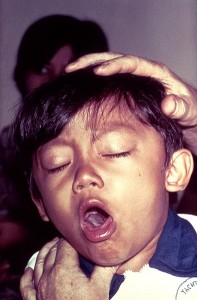 By Ijeoma Ejigiri, Class of 2011
By Ijeoma Ejigiri, Class of 2011
Faculty Peer Reviewed
Whooping cough. 100 day cough. Pertussis. These are the various names for the disease …