 By Jessica K Qiu
By Jessica K Qiu
Peer Reviewed
In 1998, there were 34 million adults aged 65 years or older in the US.1 By 2030, that number is expected to double.1 This dramatic increase …

 By Jessica K Qiu
By Jessica K Qiu
Peer Reviewed
In 1998, there were 34 million adults aged 65 years or older in the US.1 By 2030, that number is expected to double.1 This dramatic increase …
 By Matthew Vorsanger MD, David Kudlowitz MD, and Patrick Cocks MD
By Matthew Vorsanger MD, David Kudlowitz MD, and Patrick Cocks MD
Peer Reviewed
 Learning objectives:
1. Describe the pathophysiology and clinical features of G6PD Deficiency.
2. Discuss susceptibility to favism.
3. Give a brief historical perspective …
By John Hwang MD, Cindy Fang MD, Neil Shapiro MD, Marty Fried MD || Illustration by Michael Shen MD || Audio …
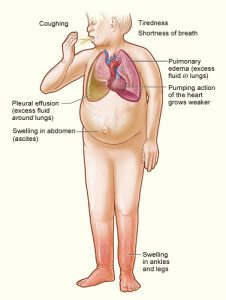 By Hannah Friedman
By Hannah Friedman
Peer Reviewed
It is a commonly seen scenario on the wards: a patient with a past medical history of heart failure and stage 4 chronic kidney disease presents with progressive …
 Join us in this episode as we question everything you ever thought you knew about… urinary tract infections (UTI) and delirium. || By Steven R. Liu MD, …
Join us in this episode as we question everything you ever thought you knew about… urinary tract infections (UTI) and delirium. || By Steven R. Liu MD, …
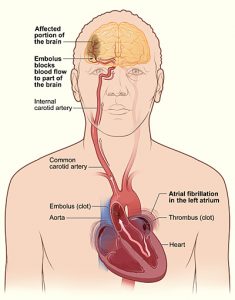 By Dixon Yang, MD
By Dixon Yang, MD
Peer ReviewedÂ
AbstractÂ
Atrial fibrillation (AF) is a common arrhythmia, especially in the elderly, and is often asymptomatic. However, absence of symptoms does not confer better prognosis. Many patients with AF …
 By Monil Shah, MD and Arun Manmadhan, MD
By Monil Shah, MD and Arun Manmadhan, MD
Peer Reviewed
A 64-year old male with a history of hypertension, dyslipidemia, and uncontrolled diabetes is brought to the emergency room with new …
 By Christopher Sonne, MD
By Christopher Sonne, MD
Peer reviewed
Learning Objectives:
1. Understand the pathophysiology of peritonitis secondary to bowel perforation.
2. Understand how classic findings of peritonitis can be absent in some …