 By Raymond Barry
By Raymond Barry
Peer Reviewed
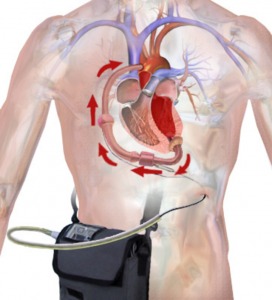
A 2020 report published by the American Heart Association (AHA) in conjunction with the National Institutes of Health (NIH) found that an estimated 6.2 million American adults had heart …

 By Raymond Barry
By Raymond Barry
Peer Reviewed
A 2020 report published by the American Heart Association (AHA) in conjunction with the National Institutes of Health (NIH) found that an estimated 6.2 million American adults had heart …
 By: Michael Moore
By: Michael Moore
Peer Reviewed
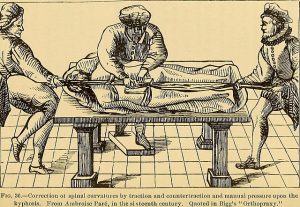
“Too many complex back surgeries are being performed and patients are suffering as a result†wrote National Public Radio health science journalist Joanne Silberner in her 2010 article “Surgery May …
 By Kathryn Hockemeyer
By Kathryn Hockemeyer
Peer Reviewed
I caught up with a friend who works in environmental, social, and corporate governance investing during a lull in the COVID-19 pandemic. Seconds into the conversation, he asked, …
 By Akshay N. Pulavarty MPH
By Akshay N. Pulavarty MPH
Peer Reviewed
A quick look into the medicine cabinet of anyone sixty or older will likely reveal a statin. Primary prevention with high-intensity statins has substantially reduced the …
 By Mahip Grewal
By Mahip Grewal
Peer Reviewed
Nonalcoholic fatty liver disease (NAFLD) is estimated to affect 25% of the world’s population.1 NAFLD is a spectrum of disease ranging from nonalcoholic fatty liver (NAFL), which is …
 By Matthew Haller
By Matthew Haller
Peer ReviewedÂ
The default mode of medical practice is curative: pursuit of the complete eradication of the malady at hand, leading to symptomatic relief and prolongation of life. In actuality, …
 By Devon Zander
By Devon Zander
Peer Reviewed
Much has been said about the art of taking a patient history. The aphorism most commonly invoked by medical educators is that “80% of diagnoses can be made …
 By Quinn Silverglate
By Quinn Silverglate
Peer Reviewed
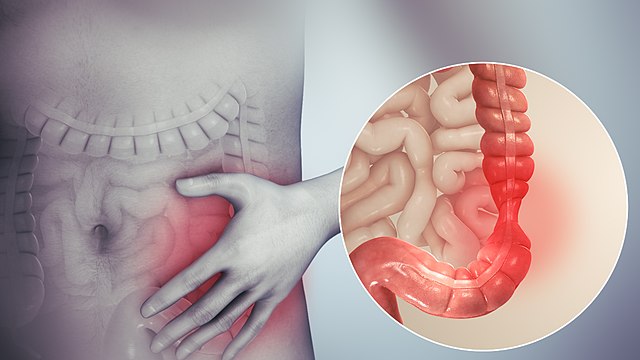
At any of the over 26,000 restaurants that populate the city of New York, I have to ask the same questions: “Does this have dairy in it? …