 By William Plowe
By William Plowe
Peer Reviewed
Metformin has been the first-line drug in type 2 diabetes for over a decade, but its possible benefit in type 1 diabetes (DM1) is still a matter of study. …

 By William Plowe
By William Plowe
Peer Reviewed
Metformin has been the first-line drug in type 2 diabetes for over a decade, but its possible benefit in type 1 diabetes (DM1) is still a matter of study. …
 By Kevin Rezzadeh
By Kevin Rezzadeh
Peer Reviewed
Injuries associated with amateur boxing include facial lacerations, hand injuries, and bruised ribs.1 While many of the superficial wounds and bone fractures can completely heal, brain …
 By Jessica K Qiu
By Jessica K Qiu
Peer Reviewed
In 1998, there were 34 million adults aged 65 years or older in the US.1 By 2030, that number is expected to double.1 This dramatic …
 By Sophia Chen
By Sophia Chen
Peer Reviewed
Once considered a disease of the West, type 2 diabetes mellitus is now a global epidemic whose incidence correlates with economic growth. This is evident in Asian countries …
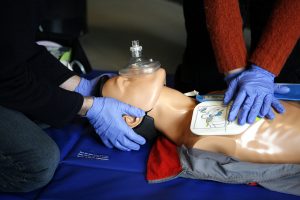 By David Pineles, MD
By David Pineles, MD
Peer Reviewed
You are a third-year internal medicine resident finishing your night shift at John Doe Hospital. Your shift so far was challenging to say …
 By Kurtis Carlock, MD
By Kurtis Carlock, MD
Peer Reviewed
Obesity is a problem that affects over one-third of adults in the US and increases the risk of numerous health problems in these individuals.[1]Â The question of how best to combat obesity is a problem that has …
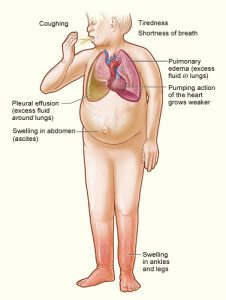 By Hannah Friedman
By Hannah Friedman
Peer Reviewed
It is a commonly seen scenario on the wards: a patient with a past medical history of heart failure and stage 4 chronic kidney disease presents with progressive …
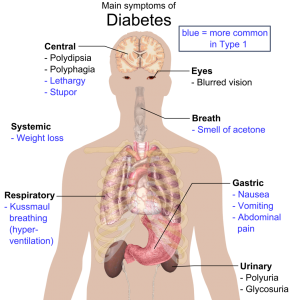 By Nicolas Gillingham
By Nicolas Gillingham
Peer Reviewed
Over 30 million Americans—9.4% of the population—live with diabetes, six million of whom are at least partially dependent on exogenous insulin.[1] Insulin can be …